Thromboembolic and Bleeding Risk in Atrial Fibrillation Patients with Chronic Kidney Disease: Role of Anticoagulation Therapy
Abstract
:1. Introduction
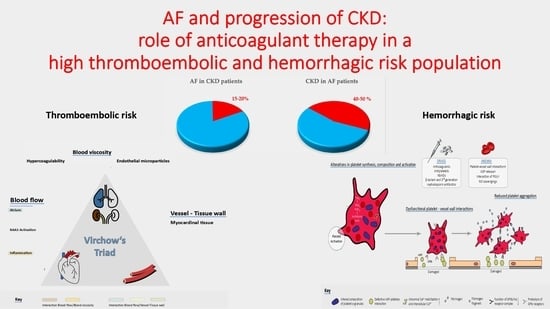
2. Pathophysiology of High Thromboembolic/Hemorrhagic Risk in CKD Patients
3. Anticoagulant-Related Nephropathy and Progression of Kidney Disease
4. DOACs, Diabetes and Chronic Kidney Disease
5. DOACs and End Stage Renal Disease
6. Non-Anticoagulative Approaches
7. Conclusions
Author Contributions
Funding
Disclosures
Conflicts of Interest
Abbreviations
| ACEi | Angiotensin converting enzyme inhibitor |
| AF | Atrial fibrillation |
| AKI | Acute kidney injury |
| ARN | Anticoagulant-related nephropathy |
| CKD | Chronic kidney disease |
| DM | Diabetes mellitus |
| DKD | Diabetic kidney disease |
| DOAC | Direct oral anticoagulant |
| eGFR | Estimated glomerular filtration rate |
| ESRD | End stage renal disease |
| INR | International normalized ratio |
| LAAO | Left atrial appendage occlusion |
| MGP | Matrix gamma-carboxyglutamate protein |
| RRT | Renal replacement therapy |
| VKA | Vitamin K antagonist |
| WRN | Warfarin-related nephropathy |
References
- Kannel, W.B.; Wolf, P.A.; Benjamin, E.J.; Levy, D. Prevalence, Incidence, Prognosis, and Predisposing Conditions for Atrial Fibrillation: Population-Based Estimates 11Reprints Are Not Available. Am. J. Cardiol. 1998, 82, 2N–9N. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, ehaa612. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turakhia, M.P.; Blankestijn, P.J.; Carrero, J.-J.; Clase, C.M.; Deo, R.; Herzog, C.A.; Kasner, S.E.; Passman, R.S.; Pecoits-Filho, R.; Reinecke, H.; et al. Chronic Kidney Disease and Arrhythmias: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur. Heart J. 2018, 39, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Banerjee, A.; Boriani, G.; Chiang, C.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef] [Green Version]
- Della Rocca, D.G.; Tarantino, N.; Trivedi, C.; Mohanty, S.; Anannab, A.; Salwan, A.S.; Gianni, C.; Bassiouny, M.; Al-Ahmad, A.; Romero, J.; et al. Non-pulmonary Vein Triggers in Nonparoxysmal Atrial Fibrillation: Implications of Pathophysiology for Catheter Ablation. J. Cardiovasc. Electrophysiol. 2020, 31, 2154–2167. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019, 140. [Google Scholar] [CrossRef]
- Kumar, S. Why Do Young People with Chronic Kidney Disease Die Early? World J. Nephrol. 2014, 3, 143. [Google Scholar] [CrossRef]
- Tonelli, M.; Muntner, P.; Lloyd, A.; Manns, B.J.; Klarenbach, S.; Pannu, N.; James, M.T.; Hemmelgarn, B.R. Risk of Coronary Events in People with Chronic Kidney Disease Compared with Those with Diabetes: A Population-Level Cohort Study. Lancet 2012, 380, 807–814. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Ananthapanyasut, W.; Napan, S.; Rudolph, E.H.; Harindhanavudhi, T.; Ayash, H.; Guglielmi, K.E.; Lerma, E.V. Prevalence of Atrial Fibrillation and Its Predictors in Nondialysis Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, E.Z.; Prineas, R.J.; Go, A.S.; Xie, D.; Lash, J.P.; Rahman, M.; Ojo, A.; Teal, V.L.; Jensvold, N.G.; Robinson, N.L.; et al. Chronic Kidney Disease and Prevalent Atrial Fibrillation: The Chronic Renal Insufficiency Cohort (CRIC). Am. Heart J. 2010, 159, 1102–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, R.G.; Eikelboom, J.W.; Brimble, K.S.; McMurtry, M.S.; Ingram, A.J. Stroke Prevention in Atrial Fibrillation Patients with Chronic Kidney Disease. Can. J. Cardiol. 2013, 29, S71–S78. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and Classification of Chronic Kidney Disease: A Position Statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, E.; Sanchez-Perales, C.; Garcia-Garcia, F.; Castellano, P.; Garcia-Cortes, M.-J.; Liebana, A.; Lozano, C. Atrial Fibrillation in Incident Dialysis Patients. Kidney Int. 2009, 76, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Genovesi, S.; Pogliani, D.; Faini, A.; Valsecchi, M.G.; Riva, A.; Stefani, F.; Acquistapace, I.; Stella, A.; Bonforte, G.; DeVecchi, A.; et al. Prevalence of Atrial Fibrillation and Associated Factors in a Population of Long-Term Hemodialysis Patients. Am. J. Kidney Dis. 2005, 46, 897–902. [Google Scholar] [CrossRef]
- Shimizu, Y.; Maeda, K.; Imano, H.; Ohira, T.; Kitamura, A.; Kiyama, M.; Okada, T.; Ishikawa, Y.; Shimamoto, T.; Yamagishi, K.; et al. Chronic Kidney Disease and Drinking Status in Relation to Risks of Stroke and Its Subtypes: The Circulatory Risk in Communities Study (CIRCS). Stroke 2011, 42, 2531–2537. [Google Scholar] [CrossRef] [Green Version]
- Bos, M.J.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M.B. Decreased Glomerular Filtration Rate Is a Risk Factor for Hemorrhagic But Not for Ischemic Stroke: The Rotterdam Study. Stroke 2007, 38, 3127–3132. [Google Scholar] [CrossRef] [Green Version]
- Iseki, K.; Kinjo, K.; Kimura, Y.; Osawa, A.; Fukiyama, K. Evidence for High Risk of Cerebral Hemorrhage in Chronic Dialysis Patients. Kidney Int. 1993, 44, 1086–1090. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppens, M.; Synhorst, D.; Eikelboom, J.W.; Yusuf, S.; Shestakovska, O.; Connolly, S.J. Efficacy and Safety of Apixaban Compared with Aspirin in Patients Who Previously Tried but Failed Treatment with Vitamin K Antagonists: Results from the AVERROES Trial. Eur. Heart J. 2014, 35, 1856–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the Efficacy and Safety of New Oral Anticoagulants with Warfarin in Patients with Atrial Fibrillation: A Meta-Analysis of Randomised Trials. Lancet 2014, 383, 955–962. [Google Scholar] [CrossRef]
- Yao, X.; Tangri, N.; Gersh, B.J.; Sangaralingham, L.R.; Shah, N.D.; Nath, K.A.; Noseworthy, P.A. Renal Outcomes in Anticoagulated Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 70, 2621–2632. [Google Scholar] [CrossRef]
- Lavalle, C.; Di Lullo, L.; Bellasi, A.; Ronco, C.; Radicchia, S.; Barbera, V.; Galardo, G.; Piro, A.; Magnocavallo, M.; Straito, M.; et al. Adverse Drug Reactions during Real-Life Use of Direct Oral Anticoagulants in Italy: An Update Based on Data from the Italian National Pharmacovigilance Network. Cardiorenal Med. 2020, 10, 266–276. [Google Scholar] [CrossRef]
- Mohanty, S.; Gianni, C.; Trivedi, C.; Gadiyaram, V.; Della Rocca, D.G.; MacDonald, B.; Horton, R.; Al-Ahmad, A.; Gibson, D.N.; Price, M.; et al. Risk of Thromboembolic Events after Percutaneous Left Atrial Appendage Ligation in Patients with Atrial Fibrillation: Long-Term Results of a Multicenter Study. Heart Rhythm 2020, 17, 175–181. [Google Scholar] [CrossRef]
- Mariani, M.V.; Magnocavallo, M.; Straito, M.; Piro, A.; Severino, P.; Iannucci, G.; Chimenti, C.; Mancone, M.; Rocca, D.G.D.; Forleo, G.B.; et al. Direct Oral Anticoagulants versus Vitamin K Antagonists in Patients with Atrial Fibrillation and Cancer a Meta-Analysis. J. Thromb. Thrombolysis 2020. [Google Scholar] [CrossRef]
- Siontis, K.C.; Zhang, X.; Eckard, A.; Bhave, N.; Schaubel, D.E.; He, K.; Tilea, A.; Stack, A.G.; Balkrishnan, R.; Yao, X.; et al. Outcomes Associated with Apixaban Use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation 2018, 138, 1519–1529. [Google Scholar] [CrossRef]
- Miao, B.; Sood, N.; Bunz, T.J.; Coleman, C.I. Rivaroxaban versus Apixaban in Non-valvular Atrial Fibrillation Patients with End-stage Renal Disease or Receiving Dialysis. Eur. J. Haematol. 2020, 104, 328–335. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.-M.; Yang, C.-W. Chronic Kidney Disease: Global Dimension and Perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Alonso, A.; Bengtson, L.G.S. A Rising Tide: The Global Epidemic of Atrial Fibrillation. Circulation 2014, 129, 829–830. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, D.; Sood, M.M.; Rigatto, C.; Holden, R.M.; Hiremath, S.; Clase, C.M. Systematic Review and Meta-Analysis of Incidence, Prevalence and Outcomes of Atrial Fibrillation in Patients on Dialysis. Nephrol. Dial. Transplant. 2012, 27, 3816–3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccini, J.P.; Stevens, S.R.; Chang, Y.; Singer, D.E.; Lokhnygina, Y.; Go, A.S.; Patel, M.R.; Mahaffey, K.W.; Halperin, J.L.; Breithardt, G.; et al. Renal Dysfunction as a Predictor of Stroke and Systemic Embolism in Patients with Nonvalvular Atrial Fibrillation: Validation of the R 2 CHADS 2 Index in the ROCKET AF (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk Factors In Atrial Fibrillation) Study Cohorts. Circulation 2013, 127, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Providência, R.; Fernandes, A.; Paiva, L.; Faustino, A.; Barra, S.; Botelho, A.; Trigo, J.; Nascimento, J.; Leitão-Marques, A. Decreased Glomerular Filtration Rate and Markers of Left Atrial Stasis in Patients with Nonvalvular Atrial Fibrillation. Cardiology 2013, 124, 3–10. [Google Scholar] [CrossRef]
- Kizawa, S.; Ito, T.; Akamatsu, K.; Ichihara, N.; Nogi, S.; Miyamura, M.; Kanzaki, Y.; Sohmiya, K.; Hoshiga, M. Chronic Kidney Disease as a Possible Predictor of Left Atrial Thrombogenic Milieu Among Patients with Nonvalvular Atrial Fibrillation. Am. J. Cardiol. 2018, 122, 2062–2067. [Google Scholar] [CrossRef]
- Yagishita, A.; Takahashi, Y.; Takahashi, A. Relationship between Transesophageal Echocar- Diographic Features and Glomerular Filtration Rate in Patients with Persistent Atrial Fibrillation. Heart Rhythm 2010, 7, S387. [Google Scholar]
- Gedikli, Ö.; Mohanty, S.; Trivedi, C.; Gianni, C.; Chen, Q.; Della Rocca, D.G.; Burkhardt, J.D.; Sanchez, J.E.; Hranitzky, P.; Gallinghouse, G.J.; et al. Impact of Dense “Smoke” Detected on Transesophageal Echocardiography on Stroke Risk in Patients with Atrial Fibrillation Undergoing Catheter Ablation. Heart Rhythm 2019, 16, 351–357. [Google Scholar] [CrossRef]
- Wang, M.-C.; Tsai, W.-C.; Chen, J.-Y.; Huang, J.-J. Stepwise Increase in Arterial Stiffness Corresponding with the Stages of Chronic Kidney Disease. Am. J. Kidney Dis. 2005, 45, 494–501. [Google Scholar] [CrossRef]
- Bolton, C.H.; Downs, L.G.; Victory, J.G.; Dwight, J.F.; Tomson, C.R.; Mackness, M.I.; Pinkney, J.H. Endothelial Dysfunction in Chronic Renal Failure: Roles of Lipoprotein Oxidation and pro-Inflammatory Cytokines. Nephrol. Dial. Transplant. 2001, 16, 1189–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heintz, B.; Schmidt, P.; Maurin, N.; Kirsten, R.; Nelson, K.; Wieland, D.; Sieberth, H.-G. Endothelin-1 Potentiates ADP-Induced Platelet Aggregation in Chronic Renal Failure. Ren. Fail. 1994, 16, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, D.G.; Pepine, C.J. Some Thoughts on the Continuing Dilemma of Angina Pectoris. Eur. Heart J. 2014, 35, 1361–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Rocca, D.G.; Pepine, C.J. Endothelium as a Predictor of Adverse Outcomes: Endothelium as a Predictor of Adverse Outcomes. Clin. Cardiol. 2010, 33, 730–732. [Google Scholar] [CrossRef]
- Tomura, S.; Nakamura, Y.; Doi, M.; Ando, R.; Ida, T.; Chida, Y.; Ootsuka, S.; Shinoda, T.; Yanagi, H.; Tsuchiya, S.; et al. Fibrinogen, Coagulation Factor VII, Tissue Plasminogen Activator, Plasminogen Activator Inhibitor-1, and Lipid as Cardiovascular Risk Factors in Chronic Hemodialysis and Continuous Ambulatory Peritoneal Dialysis Patients. Am. J. Kidney Dis. 1996, 27, 848–854. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Fried, L.F.; Crump, C.; Bleyer, A.J.; Manolio, T.A.; Tracy, R.P.; Furberg, C.D.; Psaty, B.M. Elevations of Inflammatory and Procoagulant Biomarkers in Elderly Persons with Renal Insufficiency. Circulation 2003, 107, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.; Rocha, S.; Rocha-Pereira, P.; Castro, E.; Reis, F.; Teixeira, F.; Miranda, V.; Do Sameiro Faria, M.; Loureiro, A.; Quintanilha, A.; et al. Cross-Talk between Inflammation, Coagulation/Fibrinolysis and Vascular Access in Hemodialysis Patients. J. Vasc. Access 2008, 9, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Keller, C.; Katz, R.; Cushman, M.; Fried, L.F.; Shlipak, M. Association of Kidney Function with Inflammatory and Procoagulant Markers in a Diverse Cohort: A Cross-Sectional Analysis from the Multi-Ethnic Study of Atherosclerosis (MESA). BMC Nephrol. 2008, 9, 9. [Google Scholar] [CrossRef]
- Kaw, D.; Malhotra, D. Platelet Dysfunction and End-Stage Renal Disease. Semin. Dial. 2006, 19, 317–322. [Google Scholar] [CrossRef]
- Boccardo, P.; Remuzzi, G.; Galbusera, M. Platelet Dysfunction in Renal Failure. Semin. Thromb. Hemost. 2004, 30, 579–589. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Tripodi, A. Hemostatic Defects in Liver and Renal Dysfunction. Hematol. Am. Soc. Hematol. Educ. Program. 2012, 2012, 168–173. [Google Scholar] [CrossRef]
- Reinecke, H.; Brand, E.; Mesters, R.; Schäbitz, W.-R.; Fisher, M.; Pavenstädt, H.; Breithardt, G. Dilemmas in the Management of Atrial Fibrillation in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2009, 20, 705–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravera, M.; Bussalino, E.; Paoletti, E.; Bellasi, A.; Di Lullo, L.; Fusaro, M. Haemorragic and Thromboembolic Risk in CKD Patients with Non Valvular Atrial Fibrillation: Do We Need a Novel Risk Score Calculator? Int. J. Cardiol. 2019, 274, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V.; Satoskar, A.; Chen, J.; Nadasdy, G.; Eagen, J.W.; Hamirani, M.; Hebert, L.; Calomeni, E.; Nadasdy, T. Acute Kidney Injury During Warfarin Therapy Associated with Obstructive Tubular Red Blood Cell Casts: A Report of 9 Cases. Am. J. Kidney Dis. 2009, 54, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.V.; Nadasdy, T.; Rovin, B.H.; Satoskar, A.A.; Nadasdy, G.M.; Wu, H.M.; Bhatt, U.Y.; Hebert, L.A. Warfarin-Related Nephropathy Occurs in Patients with and without Chronic Kidney Disease and Is Associated with an Increased Mortality Rate. Kidney Int. 2011, 80, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodsky, S.; Eikelboom, J.; Hebert, L.A. Anticoagulant-Related Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 2787–2793. [Google Scholar] [CrossRef] [Green Version]
- Piran, S.; Traquair, H.; Chan, N.; Robinson, M.; Schulman, S. Incidence and Risk Factors for Acute Kidney Injury in Patients with Excessive Anticoagulation on Warfarin: A Retrospective Study. J. Thromb. Thrombolysis 2018, 45, 557–561. [Google Scholar] [CrossRef]
- De Aquino Moura, K.B.; Behrens, P.M.P.; Pirolli, R.; Sauer, A.; Melamed, D.; Veronese, F.V.; da Silva, A.L.F.A. Anticoagulant-Related Nephropathy: Systematic Review and Meta-Analysis. Clin. Kidney J. 2019, 12, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Ware, K.; Brodsky, P.; Satoskar, A.A.; Nadasdy, T.; Nadasdy, G.; Wu, H.; Rovin, B.H.; Bhatt, U.; Von Visger, J.; Hebert, L.A.; et al. Warfarin-Related Nephropathy Modeled by Nephron Reduction and Excessive Anticoagulation. J. Am. Soc. Nephrol. 2011, 22, 1856–1862. [Google Scholar] [CrossRef] [Green Version]
- Golbin, L.; Vigneau, C.; Touchard, G.; Thervet, E.; Halimi, J.; Sawadogo, T.; Lagoutte, N.; Siohan, P.; Zagdoun, E.; Hertig, A.; et al. Warfarin-Related Nephropathy Induced by Three Different Vitamin K Antagonists: Analysis of 13 Biopsy-Proven Cases. Clin. Kidney J. 2017, 10, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Zeni, L.; Manenti, C.; Fisogni, S.; Terlizzi, V.; Verzeletti, F.; Gaggiotti, M.; Cancarini, G. Acute Kidney Injury Due to Anticoagulant-Related Nephropathy : A Suggestion for Therapy. Case Rep. Nephrol. 2020, 2020, 8952670. [Google Scholar] [CrossRef] [PubMed]
- Escoli, R.; Santos, P.; Andrade, S.; Carvalho, F. Dabigatran-Related Nephropathy in a Patient with Undiagnosed IgA Nephropathy. Case Rep. Nephrol. 2015, 2015, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.; Ware, K.; Qamri, Z.; Satoskar, A.; Wu, H.; Nadasdy, G.; Rovin, B.; Hebert, L.; Nadasdy, T.; Brodsky, S.V. Warfarin-Related Nephropathy Is the Tip of the Iceberg: Direct Thrombin Inhibitor Dabigatran Induces Glomerular Hemorrhage with Acute Kidney Injury in Rats. Nephrol. Dial. Transplant. 2014, 29, 2228–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Gu, Z.-C.; Ding, Z.; Shen, L.; Pan, M.-M.; Zheng, Y.-L.; Lin, H.-W.; Pu, J. Decreased Risk of Renal Impairment in Atrial Fibrillation Patients Receiving Non-Vitamin K Antagonist Oral Anticoagulants: A Pooled Analysis of Randomized Controlled Trials and Real-World Studies. Thromb. Res. 2019, 174, 16–23. [Google Scholar] [CrossRef]
- Chan, Y.-H.; See, L.-C.; Tu, H.-T.; Yeh, Y.-H.; Chang, S.-H.; Wu, L.-S.; Lee, H.-F.; Wang, C.-L.; Kuo, C.-F.; Kuo, C.-T. Efficacy and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Asians With Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, P.A.; Faus, S.A.; Williamson, M.K. Warfarin Causes Rapid Calcification of the Elastic Lamellae in Rat Arteries and Heart Valves. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1400–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennenberg, R.J.M.W.; van Varik, B.J.; Schurgers, L.J.; Hamulyak, K.; ten Cate, H.; Leiner, T.; Vermeer, C.; de Leeuw, P.W.; Kroon, A.A. Chronic Coumarin Treatment Is Associated with Increased Extracoronary Arterial Calcification in Humans. Blood 2010, 115, 5121–5123. [Google Scholar] [CrossRef] [Green Version]
- Della Rocca, D.G.; Santini, L.; Forleo, G.B.; Sanniti, A.; Del Prete, A.; Lavalle, C.; Di Biase, L.; Natale, A.; Romeo, F. Novel Perspectives on Arrhythmia-Induced Cardiomyopathy: Pathophysiology, Clinical Manifestations and an Update on Invasive Management Strategies. Cardiol. Rev. 2015, 23, 135–141. [Google Scholar] [CrossRef]
- Tantisattamo, E.; Han, K.H.; O’Neill, W.C. Increased Vascular Calcification in Patients Receiving Warfarin. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Han, K.H.; O’Neill, W.C. Increased Peripheral Arterial Calcification in Patients Receiving Warfarin. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Montone, R.A.; Niccoli, G.; Tufaro, V.; Minelli, S.; Russo, M.; Vergni, F.; Sommariva, L.; Pelliccia, F.; Bedogni, F.; Crea, F. Changes in Renal Function and Occurrence of Contrast-Induced Nephropathy after Percutaneous Coronary Interventions in Patients with Atrial Fibrillation Treated with Non-Vitamin K Oral Anticoagulants or Warfarin. Postepy W Kardiologii Interwencyjnej Adv. Interv. Cardiol. 2019, 15, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Inselman, J.W.; Ross, J.S.; Izem, R.; Graham, D.J.; Martin, D.B.; Thompson, A.M.; Ross Southworth, M.; Siontis, K.C.; Ngufor, C.G.; et al. Comparative Effectiveness and Safety of Oral Anticoagulants Across Kidney Function in Patients with Atrial Fibrillation. Circ. Cardiovasc. Qual. Outcomes 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Bohula, E.A.; Giugliano, R.P.; Ruff, C.T.; Kuder, J.F.; Murphy, S.A.; Antman, E.M.; Braunwald, E. Impact of Renal Function on Outcomes with Edoxaban in the ENGAGE AF-TIMI 48 Trial. Circulation 2016, 134, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Bisson, A.; Clementy, N.; Vourc’h, P.; Angoulvant, D.; Babuty, D.; Halimi, J.M.; Lip, G.Y.H. Changes in Glomerular Filtration Rate and Outcomes in Patients with Atrial Fibrillation. Am. Heart J. 2018, 198, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, A.; Fauchier, L.; Vourc’h, P.; Andres, C.R.; Taillandier, S.; Halimi, J.M.; Lip, G.Y.H. A Prospective Study of Estimated Glomerular Filtration Rate and Outcomes in Patients with Atrial Fibrillation: The Loire Valley Atrial Fibrillation Project. Chest 2014, 145, 1370–1382. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Abensur, H.; Betônico, C.C.R.; Machado, A.D.; Parente, E.B.; Queiroz, M.; Salles, J.E.N.; Titan, S.; Vencio, S. Interactions between Kidney Disease and Diabetes: Dangerous Liaisons. Diabetol. Metab. Syndr. 2016, 8, 50. [Google Scholar] [CrossRef]
- United States Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; United States Renal Data System: Bethesda, MD, USA, 2019. [Google Scholar]
- Fox, C.S. Predictors of New-Onset Kidney Disease in a Community-Based Population. JAMA 2004, 291, 844. [Google Scholar] [CrossRef] [Green Version]
- De Boer, I.H. Temporal Trends in the Prevalence of Diabetic Kidney Disease in the United States. JAMA 2011, 305, 2532. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and Atherosclerosis: Epidemiology, Pathophysiology, and Management. JAMA 2002, 287, 2570. [Google Scholar] [CrossRef]
- Franchi, F.; James, S.K.; Ghukasyan Lakic, T.; Budaj, A.J.; Cornel, J.H.; Katus, H.A.; Keltai, M.; Kontny, F.; Lewis, B.S.; Storey, R.F.; et al. Impact of Diabetes Mellitus and Chronic Kidney Disease on Cardiovascular Outcomes and Platelet P2Y 12 Receptor Antagonist Effects in Patients with Acute Coronary Syndromes: Insights from the PLATO Trial. J. Am. Heart Assoc. 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Ferreiro, J.L.; Angiolillo, D.J. Diabetes and Antiplatelet Therapy in Acute Coronary Syndrome. Circulation 2011, 123, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Bonello, L.; Angiolillo, D.J.; Aradi, D.; Sibbing, D. P2Y12-ADP Receptor Blockade in Chronic Kidney Disease Patients with Acute Coronary Syndromes. Circulation 2018, 138, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Chandrasekhar, J.; Sartori, S.; Aquino, M.; Kini, A.S.; Kapadia, S.; Weintraub, W.; Muhlestein, J.B.; Vogel, B.; Faggioni, M.; et al. Associations Between Chronic Kidney Disease and Outcomes with Use of Prasugrel Versus Clopidogrel in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2017, 10, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.J.; Spoendlin, J.; Mogun, H.; Gagne, J.J. Contemporary Time Trends in Use of Antiplatelet Agents Among Patients with Acute Coronary Syndrome and Comorbid Diabetes Mellitus or Chronic Kidney Disease. Pharmacotherapy 2017, 37, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kalantar-Zadeh, K. Haemodialysis-Induced Hypoglycaemia and Glycaemic Disarrays. Nat. Rev. Nephrol. 2015, 11, 302–313. [Google Scholar] [CrossRef]
- Meyer, C.; Gerich, J.E. Role of the Kidney in Hyperglycemia in Type 2 Diabetes. Curr. Diab. Rep. 2002, 2, 237–241. [Google Scholar] [CrossRef]
- Ravera, M.; Bussalino, E.; Fusaro, M.; Di Lullo, L.; Aucella, F.; Paoletti, E. Systematic DOACs Oral Anticoagulation in Patients with Atrial Fibrillation and Chronic Kidney Disease: The Nephrologist’s Perspective. J. Nephrol. 2020, 33, 483–495. [Google Scholar] [CrossRef]
- Posch, F.; Ay, C.; Stöger, H.; Kreutz, R.; Beyer-Westendorf, J. Exposure to Vitamin k Antagonists and Kidney Function Decline in Patients with Atrial Fibrillation and Chronic Kidney Disease. Res. Pract. Thromb. Haemost. 2019, 3, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Fusaro, M.; Gallieni, M.; Aghi, A.; Rizzo, M.A.; Iervasi, G.; Nickolas, T.L.; Fabris, F.; Mereu, M.C.; Giannini, S.; Sella, S.; et al. Osteocalcin (Bone GLA Protein) Levels, Vascular Calcifications, Vertebral Fractures and Mortality in Hemodialysis Patients with Diabetes Mellitus. J. Nephrol. 2019, 32, 635–643. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Joosen, I.A.; Laufer, E.M.; Chatrou, M.L.L.; Herfs, M.; Winkens, M.H.M.; Westenfeld, R.; Veulemans, V.; Krueger, T.; Shanahan, C.M.; et al. Vitamin K-Antagonists Accelerate Atherosclerotic Calcification and Induce a Vulnerable Plaque Phenotype. PLoS ONE 2012, 7, e43229. [Google Scholar] [CrossRef] [Green Version]
- Price, P.A.; Fraser, J.D.; Metz-Virca, G. Molecular Cloning of Matrix Gla Protein: Implications for Substrate Recognition by the Vitamin K-Dependent Gamma-Carboxylase. Proc. Natl. Acad. Sci. USA 1987, 84, 8335–8339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatrou, M.L.L.; Winckers, K.; Hackeng, T.M.; Reutelingsperger, C.P.; Schurgers, L.J. Vascular Calcification: The Price to Pay for Anticoagulation Therapy with Vitamin K-Antagonists. Blood Rev. 2012, 26, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.D.; Ix, J.H.; Cranenburg, E.C.M.; Vermeer, C.; Whooley, M.A.; Schurgers, L.J. Association of Kidney Function and Uncarboxylated Matrix Gla Protein: Data from the Heart and Soul Study. Nephrol. Dial. Transplant. 2009, 24, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.V.; Bradley, G.; Khan, M.; Fratoni, A.; Gasparini, A.; Roman, Y.M.; Bunz, T.J.; Eriksson, D.; Meinecke, A.-K.; Coleman, C.I. Rivaroxaban Versus Warfarin and Renal Outcomes in Non-Valvular Atrial Fibrillation Patients with Diabetes. Eur. Heart J. Qual. Care Clin. Outcomes 2019. [Google Scholar] [CrossRef]
- Fordyce, C.B.; Hellkamp, A.S.; Lokhnygina, Y.; Lindner, S.M.; Piccini, J.P.; Becker, R.C.; Berkowitz, S.D.; Breithardt, G.; Fox, K.A.A.; Mahaffey, K.W.; et al. On-Treatment Outcomes in Patients with Worsening Renal Function with Rivaroxaban Compared With Warfarin: Insights From ROCKET AF. Circulation 2016, 134, 37–47. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, E.C.; Simon, D.N.; Allen, L.A.; Singer, D.E.; Fonarow, G.C.; Kowey, P.R.; Thomas, L.E.; Ezekowitz, M.D.; Mahaffey, K.W.; Chang, P.; et al. Reasons for Warfarin Discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am. Heart J. 2014, 168, 487–494. [Google Scholar] [CrossRef]
- Liesenfeld, K.-H.; Clemens, A.; Kreuzer, J.; Brueckmann, M.; Schulze, F. Dabigatran Treatment Simulation in Patients Undergoing Maintenance Haemodialysis. Thromb. Haemost. 2016, 115, 562–569. [Google Scholar] [CrossRef]
- Wang, X.; Tirucherai, G.; Marbury, T.C.; Wang, J.; Chang, M.; Zhang, D.; Song, Y.; Pursley, J.; Boyd, R.A.; Frost, C. Pharmacokinetics, Pharmacodynamics, and Safety of Apixaban in Subjects with End-Stage Renal Disease on Hemodialysis. J. Clin. Pharmacol. 2016, 56, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Mavrakanas, T.A.; Samer, C.F.; Nessim, S.J.; Frisch, G.; Lipman, M.L. Apixaban Pharmacokinetics at Steady State in Hemodialysis Patients. J. Am. Soc. Nephrol. 2017, 28, 2241–2248. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Caluwé, R.; Bailleul, E.; De Bacquer, D.; Borrey, D.; Van Vlem, B.; Vandecasteele, S.J.; Emmerechts, J. Dose-Finding Study of Rivaroxaban in Hemodialysis Patients. Am. J. Kidney Dis. 2015, 66, 91–98. [Google Scholar] [CrossRef]
- Dias, C.; Moore, K.T.; Murphy, J.; Ariyawansa, J.; Smith, W.; Mills, R.M.; Weir, M.R. Pharmacokinetics, Pharmacodynamics, and Safety of Single-Dose Rivaroxaban in Chronic Hemodialysis. Am. J. Nephrol. 2016, 43, 229–236. [Google Scholar] [CrossRef]
- See, L.-C.; Lee, H.-F.; Chao, T.-F.; Li, P.-R.; Liu, J.-R.; Wu, L.-S.; Chang, S.-H.; Yeh, Y.-H.; Kuo, C.-T.; Chan, Y.-H.; et al. Effectiveness and Safety of Direct Oral Anticoagulants in an Asian Population with Atrial Fibrillation Undergoing Dialysis: A Population-Based Cohort Study and Meta-Analysis. Cardiovasc. Drugs Ther. 2020. [Google Scholar] [CrossRef]
- Coleman, C.I.; Kreutz, R.; Sood, N.A.; Bunz, T.J.; Eriksson, D.; Meinecke, A.-K.; Baker, W.L. Rivaroxaban Versus Warfarin in Patients With Nonvalvular Atrial Fibrillation and Severe Kidney Disease or Undergoing Hemodialysis. Am. J. Med. 2019, 132, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Takagi, H.; Ando, T.; Sugiyama, T.; Miyashita, S.; Valentin, N.; Shimada, Y.J.; Kodaira, M.; Numasawa, Y.; Briasoulis, A.; et al. Oral Anticoagulation for Patients with Atrial Fibrillation on Long-Term Hemodialysis. J. Am. Coll. Cardiol. 2020, 75, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Chokesuwattanaskul, R.; Thongprayoon, C.; Tanawuttiwat, T.; Kaewput, W.; Pachariyanon, P.; Cheungpasitporn, W. Safety and Efficacy of Apixaban versus Warfarin in Patients with End-Stage Renal Disease: Meta-Analysis. Pacing Clin. Electrophysiol. 2018, 41, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Herzog, C.A.; Turakhia, M.P.; Blankestijn, P.J.; Carrero, J.-J.; Clase, C.M.; Deo, R.; Kasner, S.E.; Passman, R.S.; Pecoits-Filho, R.; et al. Chronic Kidney Disease and Arrhythmias: Highlights from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2018, 94, 231–234. [Google Scholar] [CrossRef]
- Reinecke, H.; Jürgensmeyer, S.; Engelbertz, C.; Gerss, J.; Kirchhof, P.; Breithardt, G.; Bauersachs, R.; Wanner, C. Design and Rationale of a Randomised Controlled Trial Comparing Apixaban to Phenprocoumon in Patients with Atrial Fibrillation on Chronic Haemodialysis: The AXADIA-AFNET 8 Study. BMJ Open 2018, 8, e022690. [Google Scholar] [CrossRef]
- Beinema, M.; Brouwers, J.R.B.J.; Schalekamp, T.; Wilffert, B. Pharmacogenetic Differences between Warfarin, Acenocoumarol and Phenprocoumon. Thromb. Haemost. 2008, 100, 1052–1057. [Google Scholar]
- Ufer, M. Comparative Pharmacokinetics of Vitamin K Antagonists: Warfarin, Phenprocoumon and Acenocoumarol. Clin. Pharmacokinet. 2005, 44, 1227–1246. [Google Scholar] [CrossRef]
- Di Lullo, L.; Tripepi, G.; Ronco, C.; D’Arrigo, G.; Barbera, V.; Russo, D.; Di Iorio, B.R.; Uguccioni, M.; Paoletti, E.; Ravera, M.; et al. Cardiac Valve Calcification and Use of Anticoagulants: Preliminary Observation of a Potentially Modifiable Risk Factor. Int. J. Cardiol. 2019, 278, 243–249. [Google Scholar] [CrossRef]
- Genovesi, S.; Rossi, E.; Gallieni, M.; Stella, A.; Badiali, F.; Conte, F.; Pasquali, S.; Bertoli, S.; Ondei, P.; Bonforte, G.; et al. Warfarin Use, Mortality, Bleeding and Stroke in Haemodialysis Patients with Atrial Fibrillation. Nephrol. Dial. Transplant. 2015, 30, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holbrook, A.M.; Pereira, J.A.; Labiris, R.; McDonald, H.; Douketis, J.D.; Crowther, M.; Wells, P.S. Systematic Overview of Warfarin and Its Drug and Food Interactions. Arch. Intern. Med. 2005, 165, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteve-Pastor, M.A.; Rivera-Caravaca, J.M.; Roldán-Rabadán, I.; Roldán, V.; Muñiz, J.; Raña-Míguez, P.; Ruiz-Ortiz, M.; Cequier, Á.; Bertomeu-Martínez, V.; Badimón, L.; et al. Quality of Oral Anticoagulation with Vitamin K Antagonists in ‘Real-World’ Patients with Atrial Fibrillation: A Report from the Prospective Multicentre FANTASIIA Registry. EP Eur. 2018, 20, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Korenstra, J.; Wijtvliet, E.P.J.; Veeger, N.J.G.M.; Geluk, C.A.; Bartels, G.L.; Posma, J.L.; Piersma-Wichers, M.; Van Gelder, I.C.; Rienstra, M.; Tieleman, R.G. Effectiveness and Safety of Dabigatran versus Acenocoumarol in ‘Real-World’ Patients with Atrial Fibrillation. Europace 2016, 18, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Pokorney, S.D.; Simon, D.N.; Thomas, L.; Gersh, B.J.; Hylek, E.M.; Piccini, J.P.; Peterson, E.D. Stability of International Normalized Ratios in Patients Taking Long-Term Warfarin Therapy. JAMA 2016, 316, 661. [Google Scholar] [CrossRef] [PubMed]
- Dahal, K.; Kunwar, S.; Rijal, J.; Schulman, P.; Lee, J. Stroke, Major Bleeding, and Mortality Outcomes in Warfarin Users with Atrial Fibrillation and Chronic Kidney Disease: A Meta-Analysis of Observational Studies. Chest 2016, 149, 951–959. [Google Scholar] [CrossRef]
- Martinez, C.; Katholing, A.; Wallenhorst, C.; Freedman, S.B. Therapy Persistence in Newly Diagnosed Non-Valvular Atrial Fibrillation Treated with Warfarin or NOAC. A Cohort Study. Thromb. Haemost. 2016, 115, 31–39. [Google Scholar] [CrossRef]
- Benzimra, M.; Bonnamour, B.; Duracinsky, M.; Lalanne, C.; Aubert, J.-P.; Chassany, O.; Aubin-Auger, I.; Mahé, I. Real-Life Experience of Quality of Life, Treatment Satisfaction, and Adherence in Patients Receiving Oral Anticoagulants for Atrial Fibrillation. Patient Prefer. Adherence 2018, 12, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, Y.S.; Kim, T.-H.; Cha, M.-J.; Lee, J.M.; Park, J.; Park, J.-K.; Kang, K.-W.; Shim, J.; Uhm, J.-S.; et al. A Prospective Survey of the Persistence of Warfarin or NOAC in Nonvalvular Atrial Fibrillation: A COmparison Study of Drugs for Symptom Control and Complication PrEvention of Atrial Fibrillation (CODE-AF). Korean J. Intern. Med. 2020, 35, 99–108. [Google Scholar] [CrossRef]
- Blackshear, J.L.; Odell, J.A. Appendage Obliteration to Reduce Stroke in Cardiac Surgical Patients with Atrial Fibrillation. Ann. Thorac. Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef]
- Boersma, L.V.A.; Schmidt, B.; Betts, T.R.; Sievert, H.; Tamburino, C.; Teiger, E.; Pokushalov, E.; Kische, S.; Schmitz, T.; Stein, K.M.; et al. Implant Success and Safety of Left Atrial Appendage Closure with the WATCHMAN Device: Peri-Procedural Outcomes from the EWOLUTION Registry. Eur. Heart J. 2016, 37, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; Nielsen-Kudsk, J.E.; Cruz-Gonzalez, I.; et al. Left Atrial Appendage Occlusion for Stroke Prevention in Atrial Fibrillation: Multicentre Experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016, 11, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, D.; Afzal, M.R.; Lee, R.J.; Nagaraj, H.; Tschopp, D.; Gidney, B.; Ellis, C.; Altman, E.; Lee, B.; Kar, S.; et al. Short and Long-Term Outcomes of Percutaneous Left Atrial Appendage Suture Ligation: Results from a US Multicenter Evaluation. Heart Rhythm 2016, 13, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Gianni, C.; Anannab, A.; Sahore Salwan, A.; Della Rocca, D.G.; Natale, A.; Horton, R.P. Closure of the Left Atrial Appendage Using Percutaneous Transcatheter Occlusion Devices. J. Cardiovasc. Electrophysiol. 2020, 31, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation versus Long-Term Warfarin Therapy: The PREVAIL Trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.Y.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Neuzil, P.; Huber, K.; Halperin, J.L.; Holmes, D. PROTECT AF Investigators Percutaneous Left Atrial Appendage Closure for Stroke Prophylaxis in Patients with Atrial Fibrillation: 2.3-Year Follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation 2013, 127, 720–729. [Google Scholar] [CrossRef] [Green Version]
- Gadiyaram, V.K.; Mohanty, S.; Gianni, C.; Trivedi, C.; Al-Ahmad, A.; Burkhardt, D.J.; Gallinghouse, J.G.; Hranitzky, P.M.; Horton, R.P.; Sanchez, J.E.; et al. Thromboembolic Events and Need for Anticoagulation Therapy Following Left Atrial Appendage Occlusion in Patients with Electrical Isolation of the Appendage. J. Cardiovasc. Electrophysiol. 2019, 30, 511–516. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Horton, R.P.; Di Biase, L.; Bassiouny, M.; Al-Ahmad, A.; Mohanty, S.; Gasperetti, A.; Natale, V.N.; Trivedi, C.; Gianni, C.; et al. First Experience of Transcatheter Leak Occlusion with Detachable Coils Following Left Atrial Appendage Closure. JACC Cardiovasc. Interv. 2020, 13, 306–319. [Google Scholar] [CrossRef]
- Sedaghat, A.; Vij, V.; Streit, S.R.; Schrickel, J.W.; Al-Kassou, B.; Nelles, D.; Kleinecke, C.; Windecker, S.; Meier, B.; Valglimigli, M.; et al. Incidence, Predictors, and Relevance of Acute Kidney Injury in Patients Undergoing Left Atrial Appendage Closure with Amplatzer Occluders: A Multicentre Observational Study. Clin. Res. Cardiol. 2020, 109, 444–453. [Google Scholar] [CrossRef]
- Kefer, J.; Tzikas, A.; Freixa, X.; Shakir, S.; Gafoor, S.; Nielsen-Kudsk, J.E.; Berti, S.; Santoro, G.; Aminian, A.; Landmesser, U.; et al. Impact of Chronic Kidney Disease on Left Atrial Appendage Occlusion for Stroke Prevention in Patients with Atrial Fibrillation. Int. J. Cardiol. 2016, 207, 335–340. [Google Scholar] [CrossRef]
- Luani, B.; Genz, C.; Herold, J.; Mitrasch, A.; Mitusch, J.; Wiemer, M.; Schmeißer, A.; Braun-Dullaeus, R.C.; Rauwolf, T. Cerebrovascular Events, Bleeding Complications and Device Related Thrombi in Atrial Fibrillation Patients with Chronic Kidney Disease and Left Atrial Appendage Closure with the WATCHMANTM Device. BMC Cardiovasc. Disord. 2019, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Cruz-González, I.; Trejo-Velasco, B.; Fraile, M.P.; Barreiro-Pérez, M.; González-Ferreiro, R.; Sánchez, P.L. Left Atrial Appendage Occlusion in Hemodialysis Patients: Initial Experience. Rev. Espanola Cardiol. Engl. Ed. 2019, 72, 792–793. [Google Scholar] [CrossRef]
- Genovesi, S.; Porcu, L.; Slaviero, G.; Casu, G.; Bertoli, S.; Sagone, A.; Buskermolen, M.; Pieruzzi, F.; Rovaris, G.; Montoli, A.; et al. Outcomes on Safety and Efficacy of Left Atrial Appendage Occlusion in End Stage Renal Disease Patients Undergoing Dialysis. J. Nephrol. 2020. [Google Scholar] [CrossRef]
- Xue, X.; Jiang, L.; Duenninger, E.; Muenzel, M.; Guan, S.; Fazakas, A.; Cheng, F.; Illnitzky, J.; Keil, T.; Yu, J. Impact of Chronic Kidney Disease on Watchman Implantation: Experience with 300 Consecutive Left Atrial Appendage Closures at a Single Center. Heart Vessel. 2018, 33, 1068–1075. [Google Scholar] [CrossRef] [Green Version]
- Della Rocca, D.G.; Horton, R.P.; Tarantino, N.; Van Niekerk, C.J.; Trivedi, C.; Chen, Q.; Mohanty, S.; Anannab, A.; Murtaza, G.; Akella, K.; et al. Use of a Novel Septal Occluder Device for Left Atrial Appendage Closure in Patients with Postsurgical and Postlariat Leaks or Anatomies Unsuitable for Conventional Percutaneous Occlusion. Circ. Cardiovasc. Interv. 2020, 13, e009227. [Google Scholar] [CrossRef]
- Ayhan, H.; Mohanty, S.; Gedikli, Ö.; Trivedi, C.; Canpolat, U.; Tapia, A.C.; Chen, Q.; Della Rocca, D.G.; Gianni, C.; Salwan, A.; et al. A Simple Method to Detect Leaks after Left Atrial Appendage Occlusion with Watchman. J. Cardiovasc. Electrophysiol. 2020, 31, 2338–2343. [Google Scholar] [CrossRef]








| Stage | Description | eGFR (mL/min/1.73m2) |
|---|---|---|
| 1 | Normal or High | >90 |
| 2 | Mildly decrease | 60–89 |
| 3a | Mildly to moderately decreased | 45–59 |
| 3b | Moderately to severely decreased | 30–44 |
| 4 | Severely decreased | 15–29 |
| 5 | Renal failure (ESRD) | <15 or dialysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnocavallo, M.; Bellasi, A.; Mariani, M.V.; Fusaro, M.; Ravera, M.; Paoletti, E.; Di Iorio, B.; Barbera, V.; Della Rocca, D.G.; Palumbo, R.; et al. Thromboembolic and Bleeding Risk in Atrial Fibrillation Patients with Chronic Kidney Disease: Role of Anticoagulation Therapy. J. Clin. Med. 2021, 10, 83. https://doi.org/10.3390/jcm10010083
Magnocavallo M, Bellasi A, Mariani MV, Fusaro M, Ravera M, Paoletti E, Di Iorio B, Barbera V, Della Rocca DG, Palumbo R, et al. Thromboembolic and Bleeding Risk in Atrial Fibrillation Patients with Chronic Kidney Disease: Role of Anticoagulation Therapy. Journal of Clinical Medicine. 2021; 10(1):83. https://doi.org/10.3390/jcm10010083
Chicago/Turabian StyleMagnocavallo, Michele, Antonio Bellasi, Marco Valerio Mariani, Maria Fusaro, Maura Ravera, Ernesto Paoletti, Biagio Di Iorio, Vincenzo Barbera, Domenico Giovanni Della Rocca, Roberto Palumbo, and et al. 2021. "Thromboembolic and Bleeding Risk in Atrial Fibrillation Patients with Chronic Kidney Disease: Role of Anticoagulation Therapy" Journal of Clinical Medicine 10, no. 1: 83. https://doi.org/10.3390/jcm10010083