Preparation of PVA-Based Hollow Fiber Ion-Exchange Membranes and Their Performance for Donnan Dialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hollow Fiber Type Ion-Exchange Membranes
2.2.1. Preparation of Spinning Solution
2.2.2. Spinning of Hollow Fiber Ion-Exchange Membranes
2.2.3. Cross-Linking Processes of the Hollow Fiber Type IEMs
2.3. Preparation of a Hollow Fiber Module
2.4. Morphological Observation of Hollow Fiber Membranes
2.5. Measurement of Mechanical Strength
2.6. Measurement of Membrane Water Content
2.7. Measurement of Ion Exchange Capacity (IEC)
2.8. Donnan Dialysis Experiment
3. Results and Discussion
3.1. Morphology of the Hollow Fiber Type IEMs
3.2. Water Content of the Membranes as a Function of GA Concentration
3.3. Mechanical Properties of the Membranes as a Function of GA Concentration
3.4. Ion-Exchange Capacity of the Hollow Fiber Type Ion-Exchange Membranes
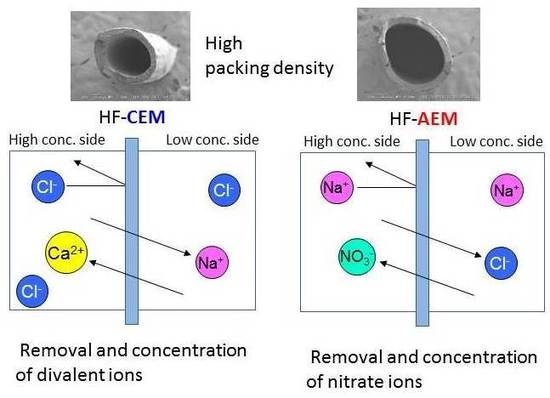
3.5. Donnan Dialysis of C-HF Membranes
3.6. Donnan Dialysis of A-HF Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sata, T. Ion Exchange Membranes: Preparation, Characterizaion, Modification and Application; Royal Society of Chemistry: London, UK, 2004; ISBN 0854045902. [Google Scholar]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Tanaka, Y. Ion Exchange Membranes Fundamentals and Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780444633194. [Google Scholar]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef] [Green Version]
- Narebska, A.; Warszawski, A. Diffusion dialysis. Effect of membrane composition on acid/salt separation. Sep. Sci. Technol. 1992, 27, 703–715. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Yamabe, T. Present status of electrodialysis in Japan. Desalination 1977, 23, 195–202. [Google Scholar] [CrossRef]
- Šímová, H.; Kysela, V.; Černín, A. Demineralization of natural sweet whey by electrodialysis at pilot-plant scale. Desalin. Water Treat. 2010, 14, 170–173. [Google Scholar] [CrossRef]
- Andres, L.J.; Riera, F.A.; Alvarez, R. Recovery and concentration by electrodialysis of tartaric acid from fruit Juice industries waste waters. J. Chem. Technol. Biotechnol. 1997, 70, 247–252. [Google Scholar] [CrossRef]
- Acheampong, M.A.; Meulepas, R.J.W.; Lens, P.N.L. Removal of heavy metals and cyanide from gold mine wastewater. J. Chem. Technol. Biotechnol. 2010, 85, 590–613. [Google Scholar] [CrossRef]
- Herz, G.P.; Byszewski, C.; Jaffari, M. Recovery of Acids and Bases Used in Metal Treatment Processes by Diffusion Dialysis; Hydrometallurgy ’94; Chapman & Hail: London, UK, 1994; pp. 613–626. [Google Scholar]
- Szczepański, P.; Szczepańska, G. Donnan dialysis—A new predictive model for non−steady state transport. J. Membr. Sci. 2017, 525, 277–289. [Google Scholar] [CrossRef]
- Akretche, D.E.; Kerdjoudj, H. Donnan dialysis of copper, gold and silver cyanides with various anion exchange membranes. Talanta 2000, 51, 281–289. [Google Scholar] [CrossRef]
- Hamouda, S.B.; Touati, K.; Amor, M.B. Donnan dialysis as membrane process for nitrate removal from drinking water: Membrane structure effect. Arab. J. Chem. 2017, 10, S287–S292. [Google Scholar] [CrossRef] [Green Version]
- Kiyono, R.; Koops, G.H.; Wessling, M.; Strathmann, H. Mixed matrix microporous hollow fibers with ion-exchange functionality. J. Membr. Sci. 2004, 231, 109–115. [Google Scholar] [CrossRef]
- Barzin, J.; Madaeni, S.S.; Pourmoghadasi, S. Hemodialysis membranes prepared from poly(vinylalcohol): Effects of the preparation conditions on the morphology and performance. J. Appl. Polym. Sci. 2007, 104, 2490–2497. [Google Scholar] [CrossRef]
- Su, B.H.; Fu, P.; Li, Q.; Tao, Y.; Li, Z.; Zao, H.S.; Zhao, C.H. Evaluation of polyethersulfone highflux hemodialysis membrane in vitro and in vivo. J. Mater. Sci. Mater. Med. 2008, 19, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Matsuyama, H.; Teramoto, M.; Nagai, H. Preparation of polymer blend hollow fiber membrane via thermally induced phase separation. Sep. Purif. Technol. 2006, 52, 363–371. [Google Scholar] [CrossRef]
- Fujiwara, N.; Matsuyama, H. The analysis and design of a both open ended hollow fiber type RO module. J. Appl. Polym. Sci. 2008, 110, 2267–2277. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Darby, J.; Bourgeous, K.; McArdle, J.; Genest, P.; Tylla, M. Ultrafiltration as an advanced tertiary treatment process for municipal wastewater. Desalination 1998, 119, 315–322. [Google Scholar] [CrossRef]
- Saito, K.; Saito, K.; Sugita, K.; Tamada, M.; Sugo, T. Cation-exchange porous hollow-fiber membranes prepared by radiation-induced cografting of GMA and EDMA which improved pure water permeability and sodium ion adsorptivity. Ind. Eng. Chem. Res. 2002, 41, 5686–5691. [Google Scholar] [CrossRef]
- Shirataki, H.; Sudoh, C.; Eshima, T.; Yokoyama, Y.; Okuyama, K. Evaluation of an anion-exchange hollow-fiber membrane adsorber containing-ray grafted glycidyl methacrylate chains. J. Chromatogr. A 2011, 1218, 2381–2388. [Google Scholar] [CrossRef]
- Wang, N.; Cui, M.; Wu, C.; Cheng, Y.; Xu, T. Hybrid Anion Exchange Hollow Fiber Membrane for Delivery of Ionic Drugs. Int. J. Chem. Eng. 2012, 2012, 832190. [Google Scholar] [CrossRef]
- Lebrum, L.; Follain, N.; Metayer, M. Elaboration of a new anion-exchange membrane with semi-interpenetrating polymer networks and characterization. Electrochim. Acta 2004, 50, 985–993. [Google Scholar] [CrossRef]
- M’Bareck, C.O.; Metayer, M.; Nguyen, Q.T.; Alexandre, S.; Malandain, J.J. Microporous anion-exchange membranes made of semi-interpenetrating polymer network I. Elaboration and characterization. J. Membr. Sci. 2003, 221, 53–68. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, J.M.; Yeon, K.H.; Moon, S.H. Electrochemical characterization of poly(vinyl alcohol)/formyl methyl pyridinium (PVA-FP) anion-exchange membranes. J. Membr. Sci. 2005, 250, 295–304. [Google Scholar] [CrossRef]
- Jikihara, A.; Ohashi, R.; Kakihana, Y.; Higa, M.; Kobayashi, K. Electrodialytic transport properties of anion-exchange membranes prepared from poly(vinyl alcohol) and poly(vinyl alcohol-co-methacryloyl aminopropyl trimethyl ammonium chloride). Membranes 2013, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hickey, A.S.; Peppas, N.A. Mesh size and diffusive characteristics of semicrystalline poly(vinyl alcohol) membranes prepared by freezing/thawing techniques. J. Membr. Sci. 1995, 107, 229–237. [Google Scholar] [CrossRef]
- Hasimi, A.; Stavropoulou, A.; Papadokostaki, K.G.; Sanopoulou, M. Transport of water in polyvinyl alcohol films: Effect of thermal treatment and chemical crosslinking. Eur. Polym. J. 2008, 44, 4098–4107. [Google Scholar] [CrossRef]
- Qin, X.H.; Wang, S.Y. Filtration Properties of Electrospinning Nanofibers. J. Appl. Polym. Sci. 2006, 10, 1285–1290. [Google Scholar] [CrossRef]
- Higa, M.; Feng, S.; Endo, N.; Kakihana, Y. Characteristics and direct methanol fuel cell performance of polymer electrolyte membranes prepared from poly(vinyl alcohol-b-styrene sulfonic acid). Electrochim. Acta 2015, 153, 83–89. [Google Scholar] [CrossRef]
- Higa, M.; Toyota, K.; Sugimoto, T. Characterization of hydrophilic hollow fiber membranes prepared from poly(vinyl alcohol). Desalin. Water Treat. 2010, 17, 199–203. [Google Scholar] [CrossRef]
- Tanaka, N.; Nagase, M.; Higa, M. Preparation of aliphatic-hydrocarbon-based anion-exchange membranes and their anti-organic-fouling properties. J. Membr. Sci. 2011, 384, 27–36. [Google Scholar] [CrossRef]
- Higa, M.; Hatemura, K.; Sugita, M.; Maesowa, S.; Nishimura, M.; Endo, N. Performance of passive direct methanol fuel cell with poly(vinyl alcohol)-based polymer electrolyte membranes. Int. J. Hydrogen Energy 2012, 37, 6292–6301. [Google Scholar] [CrossRef]
- Higa, M.; Tanioka, A.; Miyasaka, K. Simulation of the Transport of ions against their concentration gradient across charged membranes. J. Membr. Sci. 1988, 37, 251–266. [Google Scholar] [CrossRef]
- Higa, M.; Nishimura, M.; Kinoshita, K.; Jikihara, A. Characterization of cation-exchange membranes prepared from poly(vinyl alcohol) and poly(vinyl alcohol-b-styrene sulfonic acid). Int. J. Hydrogen Energy 2012, 37, 6161–6168. [Google Scholar] [CrossRef]
- Hibi, S.; Suzuki, K.; Hirano, T.; Torii, T.; Fujita, K.; Nakanishi, E.; Maeda, M. Orientation distribution function in crystallites of rolled polypropylene and poly(vinyl alcohol) films. Kobunshi Ronbunshu 1988, 45, 237–244. [Google Scholar] [CrossRef]
















| Dope solution | AP-2 and PVA/PDADMAC solution |
| Bore fluid | Water/Sodium hydrate/Sodium sulfate (83/2/15, w/w/w) |
| External coagulant | Water/Sodium hydrate/Sodium sulfate (83/2/15, w/w/w) |
| Spinneret dimensions (mm) | 2.0/0.4 (O.D./I.D.) |
| Dope solution temperature (°C) | 90 |
| Bore fluid temperature (°C) | 25 |
| External coagulant temperature (°C) | 25 |
| Bore flow rate (mL/min) | 32 |
| Air gap (cm) | 15 |
| Sample | I.D. [μm] | O.D. [μm] | d [μm] |
|---|---|---|---|
| C-HF | 910 | 1080 | 170 |
| A-HF | 1210 | 1500 | 290 |
| Sample | Theoretical IEC (meq/g) | Measured IEC (meq/g) |
|---|---|---|
| CMX | - | 1.50 |
| AMX | - | 1.40 |
| C-HF | 0.42 | 0.28 |
| A-HF | 1.25 | 0.61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higa, M.; Kakihana, Y.; Sugimoto, T.; Toyota, K. Preparation of PVA-Based Hollow Fiber Ion-Exchange Membranes and Their Performance for Donnan Dialysis. Membranes 2019, 9, 4. https://doi.org/10.3390/membranes9010004
Higa M, Kakihana Y, Sugimoto T, Toyota K. Preparation of PVA-Based Hollow Fiber Ion-Exchange Membranes and Their Performance for Donnan Dialysis. Membranes. 2019; 9(1):4. https://doi.org/10.3390/membranes9010004
Chicago/Turabian StyleHiga, Mitsuru, Yuriko Kakihana, Takehiro Sugimoto, and Kakuya Toyota. 2019. "Preparation of PVA-Based Hollow Fiber Ion-Exchange Membranes and Their Performance for Donnan Dialysis" Membranes 9, no. 1: 4. https://doi.org/10.3390/membranes9010004