Electrospun Polycaprolactone Fibrous Membranes Containing Ag, TiO2 and Na2Ti6O13 Particles for Potential Use in Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
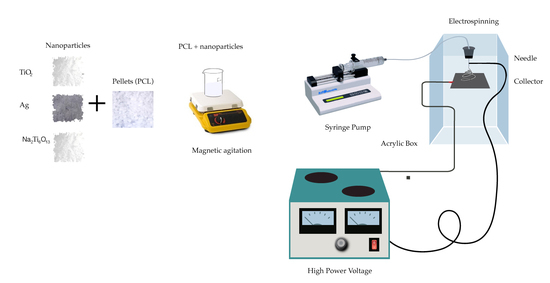
2.2. Polymer Solution Preparation
2.3. X-Ray Diffraction
2.4. Differential Scanning Calorimetry
2.5. Fourier-Transform Infrared Spectroscopy (FT–IR)
2.6. SEM and EDX
2.7. Tensile Test
2.8. Effect of Fiber in Cell Viability
2.9. Cell Adhesion
2.10. Antibacterial Assay
2.11. Bioactivity Test
3. Results and Discussion
3.1. X-Ray Diffraction
3.2. Differential Scanning Calorimetry
3.3. Fourier-Transform Infrared Spectroscopy (FT–IR)
3.4. SEM and EDX
3.5. Tensile Test
3.6. Cell Viability and Adhesion
3.7. Antibacterial Assay
3.8. Bioactivity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reddi, A.H. Morphogenesis and tissue engineering of bone and cartilage: Inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000, 6, 351–359. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Venugopal, J.; Ramakrishna, S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Wutticharoenmongkol, P.; Sanchavanakit, N.; Pavasant, P.; Supaphol, P. Preparation and characterization of novel bone scaffolds based on electrospun polycaprolactone fibers filled with nanoparticles. Macromol. Biosci. 2006, 6, 70–77. [Google Scholar] [CrossRef]
- Phipps, M.C.; Clem, W.C.; Grunda, J.M.; Clines, G.A.; Bellis, S.L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 2012, 33, 524–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.-J.; Dean, D.R.; Jun, H.-W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadegari, A.; Fahimipour, F.; Rasoulianboroujeni, M.; Dashtimoghadarm, E.; Omidi, M.; Golzar, H.; Tahriri, M.; Tayebi, L. 10—Specific considerations in scaffold design for oral tissue engineering. In Biomaterials for Oral and Dental Tissue Engineering; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 157–183. ISBN 9780081009611. [Google Scholar]
- Lee, S.-W.; Kim, S.-G. Membranes for the Guided Bone Regeneration. Maxillofacc. Plast Reconstr. Surg. 2014, 36, 239–246. [Google Scholar] [CrossRef]
- Thomas, R.; Soumya, K.R.; Mathew, J.; Radhakrishnan, E.K. Electrospun Polycaprolactone Membrane Incorporated with Biosynthesized Silver Nanoparticles as Effective Wound Dressing Material. Appl. Biochem. Biotechnol. 2015, 176, 2213–2224. [Google Scholar] [CrossRef]
- Pierchala, M.K.; Makaremi, M.; Tan, H.L.; Pushpamalar, J.; Muniyandy, S.; Solouk, A.; Lee, S.M.; Pasbakhsh, P. Nanotubes in nanofibers: Antibacterial multilayered polylactic acid/halloysite/gentamicin membranes for bone regeneration application. Appl. Clay Sci. 2018, 160, 95–105. [Google Scholar] [CrossRef]
- Herford, A.S.; Cicciù, M.; Eftimie, L.F.; Miller, M.; Signorino, F.; Famà, F.; Cervino, G.; Lo Giudice, G.; Bramanti, E.; Lauritano, F.; et al. rhBMP-2 applied as support of distraction osteogenesis: A split-mouth histological study over nonhuman primates mandibles. Int. J. Clin. Exp. Med. 2016, 9, 17187–17194. [Google Scholar]
- Chen, M.; Patra, P.K.; Warner, S.B.; Bhowmick, S. Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Eng. 2007, 13, 579–587. [Google Scholar] [CrossRef]
- Mattanavee, W.; Suwantong, O.; Puthong, S.; Bunaprasert, T.; Hoven, V.P.; Supaphol, P. Immobilization of biomolecules on the surface of electrospun polycaprolactone fibrous scaffolds for tissue engineering. ACS Appl. Mater. Interfaces 2009, 1, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Casasola, R.; Thomas, N.L.; Trybala, A.; Georgiadou, S. Electrospun poly lactic acid (PLA) fibres: Effect of different solvent systems on fibre morphology and diameter. Polymer 2014, 55, 4728–4737. [Google Scholar] [CrossRef] [Green Version]
- Koh, H.S.; Yong, T.; Chan, C.K.; Ramakrishna, S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials 2008, 29, 3574–3582. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.R.; Bhattarai, N.; Viswanathamurthi, P.; Yi, H.K.; Hwang, P.H.; Kim, H.Y. Hydrophilic nanofibrous structure of polylactide; fabrication and cell affinity. J. Biomed. Mater. Res. A 2006, 78, 247–257. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Chen, X.; Xu, Q.; Lu, F.; Nie, J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef]
- Duan, H.; Feng, B.; Guo, X.; Wang, J.; Zhao, L.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, W.J. Engineering of epidermis skin grafts using electrospun nanofibrous gelatin/ polycaprolactone membranes. Int. J. Nanomed. 2013, 8, 2077–2084. [Google Scholar]
- Lim, J.S.; Ki, C.S.; Kim, J.W.; Lee, K.G.; Kang, S.W.; Kweon, H.Y.; Park, Y.H. Fabrication and evaluation of poly(epsilon-caprolactone)/silk fibroin blend nanofibrous scaffold. Biopolymers 2012, 97, 265–275. [Google Scholar] [CrossRef]
- Aslan, S.; Loebick, C.Z.; Kang, S.; Elimelech, M.; Pfefferle, L.D.; Van Tassel, P.R. Antimicrobial biomaterials based on carbon nanotubes dispersed in poly(lactic-co-glycolic acid). Nanoscale 2010, 2, 1789–1794. [Google Scholar] [CrossRef]
- Jao, W.-C.; Yang, M.-C.; Lin, C.-H.; Hsu, C.-C. Fabrication and characterization of electrospun silk fibroin/TiO2 nanofibrous mats for wound dressings. Polym. Adv. Technol. 2012, 23, 1066–1076. [Google Scholar] [CrossRef]
- Sumitha, M.S.; Shalumon, K.T.; Sreeja, V.N.; Jayakumar, R.; Nair, S.V.; Menon, D. Biocompatible and Antibacterial Nanofibrous Poly(ε-caprolactone)-Nanosilver Composite Scaffolds for Tissue Engineering Applications. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 131–138. [Google Scholar] [CrossRef]
- Fereshteh, Z.; Fathi, M.H.; Mozaffarinia, R. Mg-doped fluorapatite nanoparticles-poly(ε-caprolactone) electrospun nanocomposite: Microstructure and mechanical properties. Superlattices Microstruct. 2014, 75, 208–221. [Google Scholar] [CrossRef]
- Lakshman, L.R.; Shalumon, K.T.; Nair, S.V.; Jayakumar, R.; Nair, S.V. Preparation of Silver Nanoparticles Incorporated Electrospun Polyurethane Nano-fibrous Mat for Wound Dressing. J. Macromol. Sci. Part A Pure Appl. Chem. 2010, 47, 1012–1018. [Google Scholar] [CrossRef]
- Vellora Thekkae Padil, V.; Nguyen, N.H.A.; Ševců, A.; Černík, M. Fabrication, Characterization, and Antibacterial Properties of Electrospun Membrane Composed of Gum Karaya, Polyvinyl Alcohol, and Silver Nanoparticles. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Karakitsou, K.E.; Verykios’, X.E. Effects of Altervalent Cation Doping of TiO2 on Its Performance as a Photocatalyst for Water Cleavage. J. Phys. Chem. 1993, 97, 1184–1189. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Y.-N.; Fu, L.; Zhang, B.; Li, F. Preparation of doped TiO2 nanofiber membranes through electrospinning and their application for photocatalytic degradation of malachite green. J. Mater. Sci. 2014, 49, 2303–2314. [Google Scholar] [CrossRef]
- Tong, H.; Tao, X.; Wu, D.; Zhang, X.; Li, D.; Zhang, L. Preparation and characterization of doped TiO2 nanofibers by coaxial electrospining combined with sol–gel process. J. Alloys Compd. 2014, 586, 274–278. [Google Scholar] [CrossRef]
- Joo Kim, H.; Raj Pant, H.; Hee Kim, J.; Jung Choi, N.; Sang Kim, C. Fabrication of multifunctional TiO2–fly ash/polyurethane nanocomposite membrane via electrospinning. Ceram. Int. 2014, 40, 3023–3029. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Marciano, S.; Pacifico, S. TiO2/PCL hybrid materials synthesized via sol–gel technique for biomedical applications. Mater. Sci. Eng. C 2015, 47, 135–141. [Google Scholar] [CrossRef]
- Ghosal, K.; Thomas, S.; Kalarikkal, N.; Gnanamani, A. Collagen coated electrospun polycaprolactone (PCL) with titanium dioxide (TiO2) from an environmentally benign solvent: Preliminary physico-chemical studies for skin substitute. J. Polym. Res. 2014, 21, 410. [Google Scholar] [CrossRef]
- Toniatto, T.V.; Rodrigues, B.V.M.; Marsi, T.C.O.; Ricci, R.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Nanostructured poly (lactic acid) electrospun fiber with high loadings of TiO2 nanoparticles: Insights into bactericidal activity and cell viability. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 381–385. [Google Scholar] [CrossRef]
- Bajsić, E.G.; Mijović, B.; Penava, N.V.; Grgurić, T.H.; Slouf, M.; Zdraveva, E. The effect of UV irradiation on the electrospun PCL/TiO2 composites fibers. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Augustine, R.; Malik, H.N.; Singhal, D.K.; Mukherjee, A.; Malakar, D.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J. Polym. Res. 2014, 21, 347. [Google Scholar] [CrossRef]
- Gupta, K.K.; Kundan, A.; Mishra, P.K.; Srivastava, P.; Mohanty, S.; Singh, N.K.; Mishra, A.; Maiti, P. Polycaprolactone composites with TiO2 for potential nanobiomaterials: Tunable properties using different phases. Phys. Chem. Chem. Phys. 2012, 14, 12844–12853. [Google Scholar] [CrossRef]
- Ghosal, K.; Manakhov, A.; Zajíčková, L.; Thomas, S. Structural and Surface Compatibility Study of Modified Electrospun Poly(ε-caprolactone) (PCL) Composites for Skin Tissue Engineering. AAPS PharmSciTech 2017, 18, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Coreño, J.; Coreño, O. Evaluation of calcium titanate as apatite growth promoter. J. Biomed. Mater. Res. A 2005, 75, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef] [Green Version]
- Kitsugi, T.; Nakamura, T.; Oka, M.; Yan, W.-Q.; Miyaji, S. Bone bonding behavior of titanium and its alloys when coated with titanium oxide (TiO2) and titanium silicate (Ti5Si3). J. Biomed. Mater. Res. 1996, 32, 149–156. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Chemical surface modification of a titanium scaffold. In Metallic Foam Bone: Processing; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Hernández, A.; Torres-Martínez, L.M.; Ortega, W.; López, T. Ceramic Compounds M2TinO2n+1 (M = Li,Na,K and n = 2), Synthesis and Photocatalytic Properties. In Emerging Fields in Sol-Gel Science and Technology; López, T.M., Avnir, D., Aegerter, M., Eds.; Springer: New York, NY, USA, 2003; pp. 84–91. ISBN 9781402074585. [Google Scholar]
- Leach, M.K.; Feng, Z.-Q.; Tuck, S.J.; Corey, J.M. Electrospinning fundamentals: Optimizing solution and apparatus parameters. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Venugopal, J.R.; Zhang, Y.; Ramakrishna, S. In Vitro Culture of Human Dermal Fibroblasts on Electrospun Polycaprolactone Collagen Nanofibrous Membrane. Artif. Organs 2006, 30, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Chazotte, B. Labeling nuclear DNA using DAPI. Cold Spring Harb. Protoc. 2011, 2011, db.prot5556. [Google Scholar] [CrossRef] [PubMed]
- Trapalis, C.C.; Keivanidis, P.; Kordas, G.; Zaharescu, M.; Crisan, M.; Szatvanyi, A.; Gartner, M. TiO2(Fe3+) nanostructured thin films with antibacterial properties. Thin Solid Films 2003, 433, 186–190. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.L.; Gomes, S.; Henriques, C.; Borges, J.P.; Silva, J.C. Electrospinning polycaprolactone dissolved in glacial acetic acid: Fiber production, nonwoven characterization, and In Vitro evaluation. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Ravelingien, M.; Mullens, S.; Luyten, J.; Meynen, V.; Vinck, E.; Vervaet, C.; Remon, J.P. Influence of Heat Treatment on the in Vitro Bioactivity of Alkali-Treated Titanium Surfaces. Ceramics-Silikaty 2010, 54, 241–247. [Google Scholar]
- Wang, F.F.; Liao, Y.M.; Wang, M.; Gong, P.; Li, X.Y.; Tang, H.; Man, Y.; Yuan, Q.; Wei, N.; Tan, Z.; et al. Evaluation of Sodium Titanate Coating on Titanium by Sol-Gel Method In Vitro. In Key Engineering Materials; Trans Tech Publ: Zurich, Switzerland, 2007; Volume 330, pp. 777–780. [Google Scholar]
- Becker, I.; Hofmann, I.; Müller, F.A. Preparation of bioactive sodium titanate ceramics. J. Eur. Ceram. Soc. 2007, 27, 4547–4553. [Google Scholar] [CrossRef]
- Jiang, S.D.; Yao, Q.Z.; Ma, Y.F.; Zhou, G.T.; Fu, S.Q. Phosphate-dependent morphological evolution of hydroxyapatite and implication for biomineralisation. Gondwana Res. 2015, 28, 858–868. [Google Scholar] [CrossRef]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef]







| Samples | tm (°C) | Tensile Strength* (MPa) | Strain at Break (%) |
|---|---|---|---|
| PCL (control) | 58.88 | 0.66 ± 0.13 | 152.40 ± 0.758 |
| PCL/Ag | 55.67 | 2.51 ± 0.13 | 901.65 ± 0.001 |
| PCL/TiO2 | 60.85 | 6.19 ± 0.72 | 190.18 ± 0.001 |
| PCL/Na2Ti6O13 | 57.87 | 1.55 ± 0.24 | 472.70 ± 0.013 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Cedillo, E.; Ortega-Lara, W.; Rocha-Pizaña, M.R.; Gutierrez-Uribe, J.A.; Elías-Zúñiga, A.; Rodríguez, C.A. Electrospun Polycaprolactone Fibrous Membranes Containing Ag, TiO2 and Na2Ti6O13 Particles for Potential Use in Bone Regeneration. Membranes 2019, 9, 12. https://doi.org/10.3390/membranes9010012
Ramírez-Cedillo E, Ortega-Lara W, Rocha-Pizaña MR, Gutierrez-Uribe JA, Elías-Zúñiga A, Rodríguez CA. Electrospun Polycaprolactone Fibrous Membranes Containing Ag, TiO2 and Na2Ti6O13 Particles for Potential Use in Bone Regeneration. Membranes. 2019; 9(1):12. https://doi.org/10.3390/membranes9010012
Chicago/Turabian StyleRamírez-Cedillo, Erick, Wendy Ortega-Lara, María R. Rocha-Pizaña, Janet A. Gutierrez-Uribe, Alex Elías-Zúñiga, and Ciro A. Rodríguez. 2019. "Electrospun Polycaprolactone Fibrous Membranes Containing Ag, TiO2 and Na2Ti6O13 Particles for Potential Use in Bone Regeneration" Membranes 9, no. 1: 12. https://doi.org/10.3390/membranes9010012