Design of a Semi-Continuous Selective Layer Based on Deposition of UiO-66 Nanoparticles for Nanofiltration
Abstract
:1. Introduction
- (i)
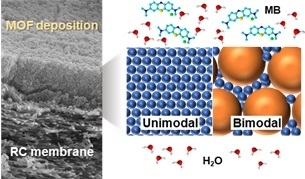
- The effect of the size and loading of the UiO-66 nanoparticles on the performance of the resultant composite membranes for the filtration of an MB aqueous solution. It was found that smaller nanoparticles yielded a selective layer superior in terms of permeability and selectivity, while a selective layer made by larger nanoparticles was more tolerant for the fouling.
- (ii)
- Design of bimodal composite membranes with the selective layer composed by a mixture of smaller and larger nanoparticles. By filling interparticle voids among the larger nanoparticles with the smaller nanoparticles, the permeability, selectivity, and tolerance for the fouling could be simultaneously improved.
2. Materials and Methods
2.1. Materials
2.2. UiO-66 Preparation and Characterization
2.3. Membrane Preparation and Characterization
2.4. Filtration Performance
3. Results
3.1. UiO-66 Characterization
3.2. Membrane Preparation and Filtration Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Shon, H.K.; Phuntsho, S.; Chaudhary, D.S.; Vigneswaran, S.; Cho, J. Nanofiltration for water and wastewater treatment—A mini review. Drink. Water Eng. Sci. 2013, 6, 47–53. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced Functional Polymer Membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric Membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [PubMed]
- Anim-Mensah, A.; Govind, R. Prediction of Polymeric Membrane Separation and Purification Performances: A Combined Mechanical, Chemical and Thermodynamic Model for Organic Systems; Springer: Berlin, Germany, 2015; pp. 5–15. [Google Scholar]
- Geise, G.M.; Park, H.B.; Sagle, A.C.; Freeman, B.D.; McGrath, J.E. Water permeability and water/salt selectivity tradeoff in polymers for desalination. J. Membr. Sci. 2011, 369, 130–138. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Mohmood, I.; Lopes, C.B.; Lopes, I.; Ahmad, I.; Duarte, A.C.; Pereira, E. Nanoscale materials and their use in water contaminants removal—A Review. Environ. Sci. Pollut. Res. 2013, 20, 1239–1260. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ahlawat, W.; Bhanjana, G.; Heydarifard, S.; Nazhad, M.M.; Dilbaghi, N. Nanotechnology-based water treatment strategies. J. Nanosci. Nanotechnol. 2014, 14, 1838–1858. [Google Scholar] [CrossRef]
- Whitby, M.; Quirke, N. Fluid flow in carbon nanotubes and nanopipes. Nat. Nanotechnol. 2007, 2, 87–94. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jiang, Y.; Gao, C. High-flux graphene oxide nanofiltration membrane intercalated by carbon nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8147–8155. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Le, L.H.; Trinh, D.X.; Trung, N.B.; Tran, T.P.N.; Taniike, T. Fabrication of assembled membrane from malonate-functionalized graphene and evaluation of its permeation performance. Carbon 2017, 114, 519–525. [Google Scholar] [CrossRef]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jiang, X.; Zhang, Y.; Lau, C.H.; Xie, Z.; Ng, D.; Smith, S.J.D.; Hill, M.R.; Shao, L. Building additional passageways in polyamide membranes with hydrostable metal organic frameworks to recycle and remove organic solutes from various solvents. ACS Appl. Mater. Interfaces 2017, 44, 38877–38886. [Google Scholar] [CrossRef]
- Lee, J.-Y.; She, Q.; Huo, F.; Tang, C.Y. Metal-organic framework-based porous matrix membranes for improving mass transfer in forward osmosis membranes. J. Membr. Sci. 2015, 492, 392–399. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T., III; et al. Tuning the structure and function of metal-organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef]
- Ma, J.; Guo, X.; Ying, Y.; Liu, D.; Zhong, C. Composite ultrafiltration membrane tailored by MOF@GO with highly improved water purification performance. Chem. Eng. J. 2017, 313, 890–898. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, Q.; Zhang, G. Metal-organic framework composite membranes: Synthesis and separation applications. Chem. Eng. Sci. 2015, 135, 232–257. [Google Scholar] [CrossRef]
- Sun, H.; Tang, B.; Wu, P. Development of hybrid ultrafiltration membranes with improved water separation properties using modified superhydrophilic metal-organic framework Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 21473–21484. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wee, L.H.; Volodin, A.; Martens, J.A.; Vankelecom, I.F.J. Polymer supported ZIF-8 membranes prepared via an interfacial synthesis method. Chem. Commun. 2015, 51, 918–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Ji, S.; Wang, N.; Wang, L.; Zhang, G.; Li, J.-R. Coordination-driven in-situ self-assembly strategy for the preparation of metal-organic framework hybrid membranes. Angew. Chem. Int. Ed. 2014, 53, 9775–9779. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; James, J.B.; Armstrong, M.R.; Close, E.C.; Letham, P.A.; Nikkhah, K.; Lin, Y.S.; Mu, B. Influences of deprotonation and modulation on nucleation and growth of UiO-66: Intergrowth and Orientation. J. Phys. Chem. C 2018, 122, 2200–2206. [Google Scholar] [CrossRef]
- Fuoco, A.; Khdhayyer, M.R.; Attfield, M.P.; Esposito, E.; Jansen, J.C.; Budd, P.M. Synthesis and transport properties of novel MOF/PIM-1/MOF sandwich membranes for gas separation. Membranes 2017, 7, 7. [Google Scholar] [CrossRef]
- Trinh, D.X.; Tran, T.P.N.; Taniike, T. Fabrication of new composite membrane filled with UiO-66 nanoparticles and its application to nanofiltration. Sep. Purif. Technol. 2017, 177, 249–256. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Demir, N.K.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Wu, F.; Lin, L.; Liu, H.; Wang, H.; Qiu, J.; Zhang, X. Synthesis of stable UiO-66 membranes for pervaporation separation of methanol/methyl tert-butyl ether mixtures by secondary growth. J. Membr. Sci. 2017, 544, 342–350. [Google Scholar] [CrossRef]
- Liu, X.; Demir, N.K.; Wu, Z.; Li, K. Highly water-stable zirconium metal-organic framework UiO-66 membranes supported on alumina hollow fibers for desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the complex structure of UiO-66 metal organic framework: A synergic combination of experiment and theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Kandiah, M.; Usseglio, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P.; Tilset, M. Post-synthetic modification of the metal-organic framework compound UiO-66. J. Mater. Chem. 2010, 20, 9848–9851. [Google Scholar] [CrossRef]
- Cliffe, M.J.; Wan, W.; Zou, X.; Chater, P.A.; Kleppe, A.K.; Tucker, M.G.; Wilhelm, H.; Funnell, N.P.; Coudert, F.-X.; Goodwin, A.L. Correlated defect nanoregions in a metal-organic frameworks. Nat. Commun. 2014, 5, 4176–4178. [Google Scholar] [CrossRef] [PubMed]
- DeCoste, J.B.; Peterson, G.W.; Schindler, B.J.; Killops, K.L.; Browe, M.A.; Mahle, J.J. The effect of water adsorption on the structure of the carboxylate containing metal-organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66. J. Mater. Chem. A 2013, 1, 11922–11932. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W.; Jasuja, H.; Glover, T.G.; Huang, Y.; Walton, K.S. Stability and degradation mechanisms of metal-organic frameworks containing the Zr6O4(OH)4 secondary building unit. J. Mater. Chem. A 2013, 1, 5642–5650. [Google Scholar] [CrossRef]
- Ragon, F.; Horcajada, P.; Chevreau, H.; Hwang, Y.K.; Lee, U.-H.; Miller, S.R.; Devic, T.; Chang, J.-S.; Serre, C. In-situ energy-dispersive X-ray diffraction for the synthesis optimization and scale-up of the porous zirconium terephthalate UiO-66. Inorg. Chem. 2014, 53, 2491–2500. [Google Scholar] [CrossRef]
- de Souza Macedo, J.; da Costa Júnior, N.B.; Almeida, L.E.; da Silva Vieira, E.F.; Cestari, A.R.; de Fátima Gimenez, I.; Carreño, N.L.V.; Barreto, L.S. Kinetic and calorimetric study of the adsorption of dyes on mesoporous activated carbon prepared from coconut coir dust. J. Colloid Interface Sci. 2006, 298, 515–522. [Google Scholar] [CrossRef]
- Lennox, M.J.; Düren, T. Understanding the kinetic and thermodynamic origins of Xylene separation in UiO-66(Zr) via molecular simulation. J. Phys. Chem. C 2016, 120, 18651–18658. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.-Y.; Chung, T.-S. Ultrathin polyamide membranes fabricated from freestanding interfacial polymerization: Synthesis, modifications, and post-treatment. Ind. Eng. Chem. Res. 2017, 56, 513–523. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, L.; Uliana, A.; Hou, J.; Wang, J.; Zhang, Y.; Li, X.; Yuan, S.; Li, J.; Tian, M.; et al. Elevated performance of thin film nanocomposite membranes enabled by modified hydrophilic MOFs for nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- de Souza Araki, M.; de Morais Coutinho, C.; Gonçalves, L.A.G.; Viotto, L.A. Solvent permeability in commercial ultrafiltration polymeric membranes and evaluation of the structural and chemical stability towards hexane. Sep. Purif. Technol. 2010, 71, 13–21. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Flux decline during nanofiltration of organic components in aqueous solution. Environ. Sci. Technol. 2001, 35, 3535–3540. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, W.-N.; Liu, D.; Nie, Y.; Li, W.; Wu, J.; Zhang, F.; Biswas, P.; Fortner, J.D. Engineered crumpled graphene oxide nanocomposite membrane assemblies for advanced water treatment processes. Environ. Sci. Technol. 2015, 49, 6846–6854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Deng, C.-H.; Yang, H.-C.; Liu, H.-Y.; Huan, S.-Y. Cross-linking to prepare composite graphene oxide-framework membranes with high-flux for dyes and heavy metal ions removal. Chem. Eng. J. 2017, 322, 657–666. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, L.; Wang, Y.; Li, R.; Gu, X.; Yuan, Y.D.; Qian, Y.; Hu, Z.; Zhao, D. Improving water-treatment performance of zirconium metal-organic framework membranes by postsynthetic defect healing. ACS Appl. Mater. Interfaces 2017, 9, 37848–37855. [Google Scholar] [CrossRef]
- Cao, J.; Su, Y.; Liu, Y.; Guan, J.; He, M.; Zhang, R.; Jiang, Z. Self-assembled MOF membranes with underwater superoleophobicity for oil/water separation. J. Membr. Sci. 2018, 566, 268–277. [Google Scholar] [CrossRef]
- Ang, H.; Hong, L. Polycationic Polymer-regulated assembling of 2D MOF nanosheets for high-performance nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 28079–28088. [Google Scholar] [CrossRef]
- Meng, L.; Lu, P.; Li, S. Packing properties of binary mixtures in disordered sphere systems. Particuology 2014, 16, 155–166. [Google Scholar] [CrossRef]








| Sample | Water Amount 1 (Molar Ratio) | Crystallite Size from XRD 2 (nm) | Particle Size from TEM 3 (nm) | Organic Content 4 (wt%) | Ligand per Zr6O4(OH)4 Cluster |
|---|---|---|---|---|---|
| UiO1 | 68 (2.0 mL) | 25 (18) | 14 | 54.09 | 11 |
| UiO2 | 55 (1.6 mL) | 37 (21) | 20 | 51.84 | 10 |
| UiO3 | 27 (0.8 mL) | 46 (37) | 75 | 51.08 | 10 |
| UiO4 | 14 (0.4 mL) | 66 (51) | 88 | 46.28 | 8 |
| UiO5 | 6.8 (0.2 mL) | 83 (52) | 108 | 46.83 | 9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shangkum, G.Y.; Chammingkwan, P.; Trinh, D.X.; Taniike, T. Design of a Semi-Continuous Selective Layer Based on Deposition of UiO-66 Nanoparticles for Nanofiltration. Membranes 2018, 8, 129. https://doi.org/10.3390/membranes8040129
Shangkum GY, Chammingkwan P, Trinh DX, Taniike T. Design of a Semi-Continuous Selective Layer Based on Deposition of UiO-66 Nanoparticles for Nanofiltration. Membranes. 2018; 8(4):129. https://doi.org/10.3390/membranes8040129
Chicago/Turabian StyleShangkum, Goji Y., Patchanee Chammingkwan, Dai X. Trinh, and Toshiaki Taniike. 2018. "Design of a Semi-Continuous Selective Layer Based on Deposition of UiO-66 Nanoparticles for Nanofiltration" Membranes 8, no. 4: 129. https://doi.org/10.3390/membranes8040129