Surface Modification of UiO-66 on Hollow Fibre Membrane for Membrane Distillation
Abstract
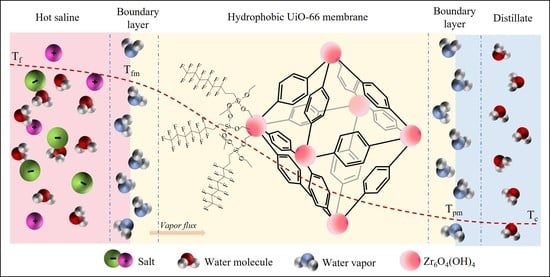
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Alumina Hollow Fibre Membranes
2.3. Functionalisation of Alumina Hollow Fibre Membranes
2.3.1. Seeding Growth of ZrO2
2.3.2. In Situ Solvothermal Synthesis of UiO-66
2.3.3. Hydrophobisation of Membrane
2.4. Characterisations
2.5. Filtration Performance in the Membrane Distillation System
3. Results and Discussion
3.1. Properties of Alumina Hollow Fibre
3.2. Physical Characterisation of the Membranes
3.3. Surface Wettability
3.4. Chemical Composition and Interaction of Modified Membranes
3.5. Desalination Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Security, W. IDA Water Security Handbook 2019–2020; Media Analytics Ltd.: Oxford, UK, 2020; ISBN 9781907467578. [Google Scholar]
- Wang, L.K.; Chen, J.P.; Hung, Y.-T.; Shammas, N.K. Membrane and Desalination Technologies; Springer: Berlin/Heidelberg, Germany, 2008; Volume 13. [Google Scholar]
- Okampo, E.J.; Nwulu, N. Optimisation of renewable energy powered reverse osmosis desalination systems: A state-of-the-art review. Renew. Sustain. Energy Rev. 2021, 140, 110712. [Google Scholar] [CrossRef]
- Park, K.; Davies, P.A. A compact hybrid batch/semi-batch reverse osmosis (HBSRO) system for high-recovery, low-energy desalination. Desalination 2021, 504, 114976. [Google Scholar] [CrossRef]
- Liu, M.; He, Q.; Guo, Z.; Zhang, K.; Yu, S.; Gao, C. Composite reverse osmosis membrane with a selective separation layer of double-layer structure for enhanced desalination, anti-fouling and durability properties. Desalination 2021, 499, 114838. [Google Scholar] [CrossRef]
- Ahmed, D.F.; Isawi, H.; Badway, N.A.; Elbayaa, A.A.; Shawky, H. Graphene oxide incorporated cellulose triacetate/cellulose acetate nanocomposite membranes for forward osmosis desalination. Arab. J. Chem. 2021, 14, 102995. [Google Scholar] [CrossRef]
- Khazaie, F.; Shokrollahzadeh, S.; Bide, Y.; Sheshmani, S.; Shahvelayati, A.S. Forward osmosis using highly water dispersible sodium alginate sulfate coated-Fe3O4 nanoparticles as innovative draw solution for water desalination. Process Saf. Environ. Prot. 2021, 146, 789–799. [Google Scholar] [CrossRef]
- Belgada, A.; Achiou, B.; Younssi, S.A.; Charik, F.Z.; Ouammou, M.; Cody, J.A.; Benhida, R.; Khaless, K. Low-cost ceramic microfiltration membrane made from natural phosphate for pretreatment of raw seawater for desalination. J. Eur. Ceram. Soc. 2021, 41, 1613–1621. [Google Scholar] [CrossRef]
- Chang, H.; Li, T.; Liu, B.; Chen, C.; He, Q.; Crittenden, J.C. Smart ultrafiltration membrane fouling control as desalination pretreatment of shale gas fracturing wastewater: The effects of backwash water. Environ. Int. 2019, 130, 104869. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yu, X.; Da, X.; Qiu, M.; Chen, X.; Fan, Y. Fabrication of a charged PDA/PEI/Al2O3 composite nanofiltration membrane for desalination at high temperatures. Sep. Purif. Technol. 2021, 263, 118388. [Google Scholar] [CrossRef]
- Yadav, V.; Rathod, N.H.; Sharma, J.; Kulshrestha, V. Long side-chain type partially cross-linked poly (vinylidene fluoride-co-hexafluoropropylene) anion exchange membranes for desalination via electrodialysis. J. Memb. Sci. 2021, 622, 119034. [Google Scholar] [CrossRef]
- Pan, J.; Wei, B.; Xie, H.; Feng, J.; Liao, S.; Li, X.; Yu, Y. Hexyl-modified series-connected bipyridine and DABCO di-cations functionalized anion exchange membranes for electrodialysis desalination. Sep. Purif. Technol. 2021, 265, 118526. [Google Scholar] [CrossRef]
- Andrés-Mañas, J.A.; Roca, L.; Ruiz-Aguirre, A.; Acién, F.G.; Gil, J.D.; Zaragoza, G. Application of solar energy to seawater desalination in a pilot system based on vacuum multi-effect membrane distillation. Appl. Energy 2020, 258, 114068. [Google Scholar] [CrossRef]
- Al-Obaidani, S.; Curcio, E.; Macedonio, F.; Di Profio, G.; Al-Hinai, H.; Drioli, E. Potential of membrane distillation in seawater desalination: Thermal efficiency, sensitivity study and cost estimation. J. Memb. Sci. 2008, 323, 85–98. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Dindi, A.; Balogun, H.A. Polygeneration in desalination by photovoltaic thermal systems: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 130, 109946. [Google Scholar] [CrossRef]
- Lee, K.P. Fabrication and Applications of Nanoporous Alumina Membranes. Ph.D. Thesis, University of Bath, Bath, UK, 2013. [Google Scholar]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, e427. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Baabbad, M.; Chung, Y.T.; Mahmoudi, E. Influence of Metal Oxide Nanoparticles in Membranes for Water Treatment and Desalination. In Proceedings of the Qatar Foundation Annual Research Conference Proceedings Volume 2014 Issue 1, Doha, Qatar, 18–19 November 2014; p. EEPP0899. [Google Scholar]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Xiao, X.; Klomkliang, N.; Yusubov, M.S.; Verpoort, F. Tunable metal–organic frameworks for heat transformation applications. Nanomaterials 2018, 8, 661. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W.; Jasuja, H.; Glover, T.G.; Huang, Y.; Walton, K.S. Stability and degradation mechanisms of metal–organic frameworks containing the Zr6O4(OH)4 secondary building unit. J. Mater. Chem. A 2013, 1, 5642–5650. [Google Scholar] [CrossRef]
- Picard, C.; Larbot, A.; Guida-Pietrasanta, F.; Boutevin, B.; Ratsimihety, A. Grafting of ceramic membranes by fluorinated silanes: Hydrophobic features. Sep. Purif. Technol. 2001, 25, 65–69. [Google Scholar] [CrossRef]
- Xu, W.-T.; Zhao, Z.-P.; Liu, M.; Chen, K.-C. Morphological and hydrophobic modifications of PVDF flat membrane with silane coupling agent grafting via plasma flow for VMD of ethanol—Water mixture. J. Memb. Sci. 2015, 491, 110–120. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, G.; Liu, S.; Shen, J.; Jin, W. A facile way to prepare ceramic-supported graphene oxide composite membrane via silane-graft modification. Appl. Surf. Sci. 2014, 307, 631–637. [Google Scholar] [CrossRef]
- Khemakhem, S.; Amar, R. Ben Modification of Tunisian clay membrane surface by silane grafting: Application for desalination with Air Gap Membrane Distillation process. Colloids Surf. A Physicochem. Eng. Asp. 2011, 387, 79–85. [Google Scholar] [CrossRef]
- Liu, X.; Demir, N.K.; Wu, Z.; Li, K. Highly water-stable zirconium metal–organic framework UiO-66 membranes supported on alumina hollow fibers for desalination. J. Am. Chem. Soc. 2015, 137, 6999–7002. [Google Scholar] [CrossRef]
- Miyamoto, M.; Hori, K.; Goshima, T.; Takaya, N.; Oumi, Y.; Uemiya, S. An organoselective zirconium-based metal–organic-framework UiO-66 membrane for pervaporation. Eur. J. Inorg. Chem. 2017, 2017, 2094–2099. [Google Scholar] [CrossRef]
- Wu, F.; Lin, L.; Liu, H.; Wang, H.; Qiu, J.; Zhang, X. Synthesis of stable UiO-66 membranes for pervaporation separation of methanol/methyl tert-butyl ether mixtures by secondary growth. J. Memb. Sci. 2017, 544, 342–350. [Google Scholar] [CrossRef]
- Trinh, D.X.; Tran, T.P.N.; Taniike, T. Fabrication of new composite membrane filled with UiO-66 nanoparticles and its application to nanofiltration. Sep. Purif. Technol. 2017, 177, 249–256. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, G.; Wang, L.; Li, J.; An, Q.; Ji, S. Pervaporation dehydration of acetic acid using NH2-UiO-66/PEI mixed matrix membranes. Sep. Purif. Technol. 2017, 186, 20–27. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Li, G.; Li, H. Preparation and characterization of alumina hollow fiber membranes. Front. Chem. Eng. China 2009, 3, 265–271. [Google Scholar] [CrossRef]
- Kingsbury, B.F.K.; Li, K. A morphological study of ceramic hollow fibre membranes. J. Memb. Sci. 2009, 328, 134–140. [Google Scholar] [CrossRef]
- Abdullah, N.; Rahman, M.A.; Othman, M.H.D.; Ismail, A.F.; Jaafar, J.; Aziz, A.A. Preparation and characterization of self-cleaning alumina hollow fiber membrane using the phase inversion and sintering technique. Ceram. Int. 2016, 42, 12312–12322. [Google Scholar] [CrossRef]
- Yahaya, N.Z.S.; Paiman, S.H.; Abdullah, N.; Mahpoz, N.M.; Raffi, A.A.; Rahman, M.A.; Abas, K.H.; Aziz, A.A.; Othman, M.H.D.; Jaafar, J. Synthesis and characterizations of MIL-140B-Al2O3/YSZ ceramic membrane using solvothermal method for seawater desalination. J. Aust. Ceram. Soc. 2019, 56, 291–300. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kohmura, S.; Iwatsuka, H.; Oumi, Y.; Uemiya, S. In situ solvothermal growth of highly oriented Zr-based metal organic framework UiO-66 film with monocrystalline layer. CrystEngComm 2015, 17, 3422–3425. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Kadir, S.H.S.A.; Jamalludin, M.R.; Harun, Z.; Abd Aziz, M.H.; Rahman, M.A.; Jaafar, J.; Nomura, M.; Honda, S. Removal of As (III) and As (V) from water using green, silica-based ceramic hollow fibre membranes via direct contact membrane distillation. RSC Adv. 2019, 9, 3367–3376. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Memb. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Li, K. Ceramic Membranes for Separation and Reaction; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 0470319461. [Google Scholar]
- Terra, N.M.; Lemos, C.O.T.; Da Silva, F.B.; Cardoso, V.L.; Reis, M.H.M. Characterisation of asymmetric alumina hollow fibres: Application for hydrogen permeation in composite membranes. Braz. J. Chem. Eng. 2016, 33, 567–576. [Google Scholar] [CrossRef]
- Liu, X.; Jin, J.; Meng, H. In situ Growth of UiO-66 with Its Particle Size Reduced by 90% into Porous Polyacrylate: Experiments and Applications. Ind. Eng. Chem. Res. 2022, 61, 7902–7910. [Google Scholar] [CrossRef]
- Ghalei, B.; Wakimoto, K.; Wu, C.Y.; Isfahani, A.P.; Yamamoto, T.; Sakurai, K.; Higuchi, M.; Chang, B.K.; Kitagawa, S.; Sivaniah, E. Rational tuning of zirconium metal—organic framework membranes for hydrogen purification. Angew. Chem. Int. Ed. 2019, 58, 19034–19040. [Google Scholar] [CrossRef]
- Lan, X.; Huang, N.; Wang, J.; Wang, T. A general and facile strategy for precisely controlling the crystal size of monodispersed metal–organic frameworks via separating the nucleation and growth. Chem. Commun. 2018, 54, 584–587. [Google Scholar] [CrossRef]
- Wang, J.; Imaz, I.; Maspoch, D. Metal—Organic Frameworks: Why Make Them Small? Small Struct. 2022, 3, 2100126. [Google Scholar] [CrossRef]
- Koonaphapdeelert, S.; Li, K. Preparation and characterization of hydrophobic ceramic hollow fibre membrane. J. Memb. Sci. 2007, 291, 70–76. [Google Scholar] [CrossRef]
- Fang, H.; Gao, J.F.; Wang, H.T.; Chen, C.S. Hydrophobic porous alumina hollow fiber for water desalination via membrane distillation process. J. Memb. Sci. 2012, 403, 41–46. [Google Scholar] [CrossRef]
- Wei, C.C.; Li, K. Preparation and characterization of a robust and hydrophobic ceramic membrane via an improved surface grafting technique. Ind. Eng. Chem. Res. 2009, 48, 3446–3452. [Google Scholar] [CrossRef]
- Yoshida, W.; Cohen, Y. Topological AFM characterization of graft polymerized silica membranes. J. Memb. Sci. 2003, 215, 249–264. [Google Scholar] [CrossRef]
- Kujawa, J.; Rozicka, A.; Cerneaux, S.; Kujawski, W. The influence of surface modification on the physicochemical properties of ceramic membranes. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 567–575. [Google Scholar] [CrossRef]
- Feng, X.J.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Li, D.; Bie, Z. Metal-organic framework incorporated monolithic capillary for selective enrichment of phosphopeptides. RSC Adv. 2017, 7, 15894–15902. [Google Scholar] [CrossRef]
- Luan, Y.; Qi, Y.; Gao, H.; Andriamitantsoa, R.S.; Zheng, N.; Wang, G. A general post-synthetic modification approach of amino-tagged metal-organic frameworks to access efficient catalysts for the Knoevenagel condensation reaction. J. Mater. Chem. A 2015, 3, 17320–17331. [Google Scholar] [CrossRef]
- Yusof, N.F.; Mehamod, F.S.; Suah, F.B.M. Fabrication and binding characterization of ion imprinted polymers for highly selective Co2+ ions in an aqueous medium. J. Environ. Chem. Eng. 2019, 7, 103007. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef] [PubMed]
- Mahltig, B. Smart hydrophobic and soil-repellent protective composite coatings for textiles and leather. In Smart Composite Coatings and Membranes; Elsevier: Amsterdam, The Netherlands, 2016; pp. 261–292. [Google Scholar]
- Kan, C.-W. Plasma treatments for sustainable textile processing. In Sustainable Apparel; Elsevier: Amsterdam, The Netherlands, 2015; pp. 49–118. [Google Scholar]
- Bollino, F.; Armenia, E.; Tranquillo, E. Zirconia/hydroxyapatite composites synthesized via Sol-Gel: Influence of hydroxyapatite content and heating on their biological properties. Materials 2017, 10, 757. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Xie, S.; Quan, H.; Luo, X.; Guo, L. Adsorption behaviors of organic micropollutants on zirconium metal-organic framework UiO-66: Analysis of surface interactions. ACS Appl. Mater. Interfaces 2017, 9, 41043–41054. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Li, S. Preparation of fluorinated graphene to study its gas sensitivity. RSC Adv. 2018, 8, 23459–23467. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Han, Y.-J.; Seo, Y.D.; Nakabayashi, K.; Miyawaki, J.; Santamaría, R.; Menéndez, R.; Yoon, S.-H.; Jang, J. C4F8 plasma treatment as an effective route for improving rate performance of natural/synthetic graphite anodes in lithium ion batteries. Carbon 2016, 103, 28–35. [Google Scholar] [CrossRef]
- Ge, J.; Wu, Z.; Wang, Q.; Yang, S. Postsynthesis of titanium incorporated with amino-functionalization UiO-66 for enhancing CO2 uptake. Quim. Nova 2019, 42, 129–134. [Google Scholar] [CrossRef]
- Teeparthi, S.R.; Awin, E.W.; Kumar, R. Dominating role of crystal structure over defect chemistry in black and white zirconia on visible light photocatalytic activity. Sci. Rep. 2018, 8, 5541. [Google Scholar] [CrossRef]
- Feng, S.; Wang, R.; Feng, S.; Zhang, Z.; Mao, L. Synthesis of Zr-based MOF nanocomposites for efficient visible-light photocatalytic degradation of contaminants. Res. Chem. Intermed. 2019, 45, 1263–1279. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Z.; Liu, H.; Wang, Y. Cd0.2Zn0.8S@UiO-66-NH2 nanocomposites as efficient and stable visible-light-driven photocatalyst for H2 evolution and CO2 reduction. Appl. Catal. B Environ. 2017, 200, 448–457. [Google Scholar] [CrossRef]
- Bambalaza, S.E.; Langmi, H.W.; Mokaya, R.; Musyoka, N.M.; Ren, J.; Khotseng, L.E. Compaction of a zirconium metal-organic framework (UiO-66) for high density hydrogen storage applications. J. Mater. Chem. A 2018, 6, 23569–23577. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Ma, Z.; Li, W.; Zhou, Y.; Zhou, J.; Zheng, W.; Sun, C.Q. Size, separation, structural order and mass density of molecules packing in water and ice. Sci. Rep. 2013, 3, 3005. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, G.; Sun, Y.; Huang, L. A computational study of water in UiO-66 Zr-MOFs: Diffusion, hydrogen bonding network, and confinement effect. AIChE J. 2021, 67, e17035. [Google Scholar] [CrossRef]
- Connolly, B.M.; Aragones-Anglada, M.; Gandara-Loe, J.; Danaf, N.A.; Lamb, D.C.; Mehta, J.P.; Vulpe, D.; Wuttke, S.; Silvestre-Albero, J.; Moghadam, P.Z. Tuning porosity in macroscopic monolithic metal-organic frameworks for exceptional natural gas storage. Nat. Commun. 2019, 10, 2345. [Google Scholar] [CrossRef]
- Luu, C.L.; Van Nguyen, T.T.; Nguyen, T.; Hoang, T.C. Synthesis, characterization and adsorption ability of UiO-66-NH2. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 25004. [Google Scholar] [CrossRef]
- Policicchio, A.; Florent, M.; Celzard, A.; Fierro, V.; Jagiello, J.; Bandosz, T.J. Enhancing the gas adsorption capacities of UiO-66 by nanographite addition. Microporous Mesoporous Mater. 2020, 309, 110571. [Google Scholar] [CrossRef]
- Zhao, D.L.; Yeung, W.S.; Zhao, Q.; Chung, T.-S. Thin-film nanocomposite membranes incorporated with UiO-66-NH2 nanoparticles for brackish water and seawater desalination. J. Memb. Sci. 2020, 604, 118039. [Google Scholar] [CrossRef]
- Wan, L.; Zhou, C.; Xu, K.; Feng, B.; Huang, A. Synthesis of highly stable UiO-66-NH2 membranes with high ions rejection for seawater desalination. Microporous Mesoporous Mater. 2017, 252, 207–213. [Google Scholar] [CrossRef]
- Yahaya, N.Z.S.; Yusof, N.F.; Paiman, S.H.; Abdullah, N.; Makhtar, S.N.N.M.; Rahman, M.A.; Abas, K.H.; Omar, M.F.; Othman, M.H.D.; Jaafar, J. Interaction of metal organic framework with fluorinated polymer on ceramic hollow fiber. Appl. Surf. Sci. 2021, 555, 149674. [Google Scholar] [CrossRef]
















| Samples | BET Surface Area (m2/g) | BJH Pore Diameter (nm) | BJH Pore Volume (cm3/g) |
|---|---|---|---|
| UiO-66 | 1322.18 | 1.93 | 0.22 |
| UiO-66/FAS | 1094.09 | 1.92 | 0.17 |
| Membranes | LEP (bar) |
|---|---|
| Pristine AHF | 1.0 ± 0.05 |
| UiO-66 | 1.2 ± 0.1 |
| S1 | 2.4 ± 0.05 |
| S2 | 2.8 ± 0.1 |
| S3 | 4.2 ± 0.1 |
| S4 | 4.7 ± 0.05 |
| S5 | 6.1 ± 0.2 |
| U1 | 2.3 ± 0.05 |
| U2 | 3.0 ± 0.05 |
| U3 | 3.8 ± 0.3 |
| U4 | 4.5 ± 0.1 |
| U5 | 5.8 ± 0.1 |
| MOFs | Membrane Process | Membrane Conditions | Water Flux | Salt Rejection (%) | Ref. |
|---|---|---|---|---|---|
| UiO-66 | MD | Hybrid MOFs/FAS deposited on AHF | 2.65 to 14.95 L/m2∙h | NaCl = 95–99 | This work |
| UiO-66 | Dead-end filtration | MOFs deposited on AHF | 0.14 to 0.28 L/m2∙h∙bar | NaCl = 47 | [29] |
| UiO-66-NH2 | Reverse osmosis | MOFs incorporated on polyamide thin film nanocomposite | 1.88 to 3.48 L/m2∙h∙bar, 1.74 to 2.99 L/m2∙h∙bar | NaCl = 95 | [73] |
| UiO-66-NH2 | Pervaporation | MOFs deposited on 3-aminopropy-ltriethoxysilane-modified Al2O3 tube | 1.5 to 12.1 kg/m2∙h | = 99.94 = 99.98 | [74] |
| UiO-66-NDC | Forward osmosis | MOFs coated with UV curable resin and deposited on AHF | 6.0 to 16.18 L/m2∙h | NaCl = 80% | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusof, N.F.; Raffi, A.A.; Yahaya, N.Z.S.; Abas, K.H.; Othman, M.H.D.; Jaafar, J.; Rahman, M.A. Surface Modification of UiO-66 on Hollow Fibre Membrane for Membrane Distillation. Membranes 2023, 13, 253. https://doi.org/10.3390/membranes13030253
Yusof NF, Raffi AA, Yahaya NZS, Abas KH, Othman MHD, Jaafar J, Rahman MA. Surface Modification of UiO-66 on Hollow Fibre Membrane for Membrane Distillation. Membranes. 2023; 13(3):253. https://doi.org/10.3390/membranes13030253
Chicago/Turabian StyleYusof, Noor Fadilah, Amirul Afiat Raffi, Nur Zhatul Shima Yahaya, Khairul Hamimah Abas, Mohd Hafiz Dzarfan Othman, Juhana Jaafar, and Mukhlis A. Rahman. 2023. "Surface Modification of UiO-66 on Hollow Fibre Membrane for Membrane Distillation" Membranes 13, no. 3: 253. https://doi.org/10.3390/membranes13030253