Thin-Film Composite Matrimid-Based Hollow Fiber Membranes for Oxygen/Nitrogen Separation by Gas Permeation
Abstract
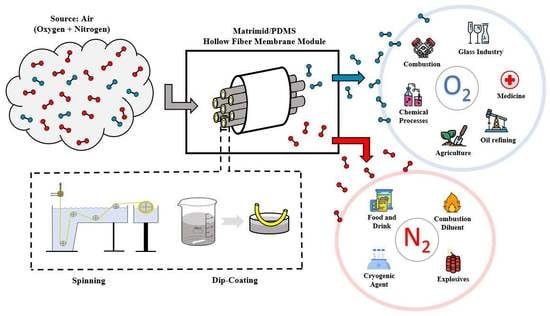
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spinning
2.3. Dip-Coating
2.4. Membrane Characterization
2.5. Gas Permeation Experiments
3. Results and Discussion
3.1. Morphology Study of Hollow Fiber Membranes
3.1.1. Morphology Study of Matrimid Hollow Fiber Membrane
3.1.2. Morphology Study of PVDF/PDMS Hollow Fiber Membrane
3.1.3. Morphology Study of Dual-Layer Hollow Fiber Membrane (Matrimid/PDMS)
3.2. Separation Performance of Membranes
3.2.1. Performance of Matrimid Hollow Fiber Membrane
3.2.2. Performance of PVDF/PDMS Hollow Fiber Membrane
3.2.3. Performance of Dual-Layer Hollow Fiber Membrane (Matrimid/PDMS)
3.3. Temperature Dependence
3.4. Comparison with Previous Studies on Gas Separation Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ismail, A.F. O2/N2 Separation. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1421–1422. [Google Scholar] [CrossRef]
- Akbari, B.; Lashanizadegan, A.; Darvishi, P.; Pouranfard, A. Preparation of Hydrophobic Flat Sheet Membranes from PVDF-HFP Copolymer for Enhancing the Oxygen Permeance in Nitrogen/Oxygen Gas Mixture. Chin. J. Chem. Eng. 2020, 28, 1566–1581. [Google Scholar] [CrossRef]
- Adhikari, B.; Orme, C.J.; Klaehn, J.R.; Stewart, F.F. Technoeconomic Analysis of Oxygen-Nitrogen Separation for Oxygen Enrichment Using Membranes. Sep. Purif. Technol. 2021, 268, 118703. [Google Scholar] [CrossRef]
- Bozorg, M.; Addis, B.; Piccialli, V.; Ramírez-Santos, Á.A.; Castel, C.; Pinnau, I.; Favre, E. Polymeric Membrane Materials for Nitrogen Production from Air: A Process Synthesis Study. Chem. Eng. Sci. 2019, 207, 1196–1213. [Google Scholar] [CrossRef]
- Zhou, H.; Cai, Y.; Xiao, Y.; Mkhalel, Z.A.; You, B.; Shi, J.; Li, J.; Chen, B.H. Process Configurations and Simulations for a Novel Single-Column Cryogenic Air Separation Process. Ind. Eng. Chem. Res. 2012, 51, 15431–15439. [Google Scholar] [CrossRef]
- Chong, K.C.; Lai, S.O.; Thiam, H.S.; Teoh, H.C.; Heng, S.L. Recent Progress of Oxygen/Nitrogen Separation Using Membrane Technology. J. Eng. Sci. Technol. 2016, 11, 1016–1030. [Google Scholar]
- Habib, M.A.; Nemitallah, M.A.; Afaneh, D.; Mezghani, K. Characteristic of Air Separation in Hollow-Fiber Polymeric Membrane for Oxygen Enriched Air Clean Combustion Applications. J. Clean. Prod. 2017, 143, 960–972. [Google Scholar] [CrossRef]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Baker, R.W. Research Needs in the Membrane Separation Industry: Looking Back, Looking Forward. J. Memb. Sci. 2010, 362, 134–136. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Sridhar, S.; Bee, S.; Bhargava, S. Membrane-Based Gas Separation: Principle, Applications and Future Potential. Chem. Eng. Dig. 2014, 1, 1–25. [Google Scholar]
- Coombe, H.S.; Nieh, S. Polymer Membrane Air Separation Performance for Portable Oxygen Enriched Combustion Applications. Energy Convers. Manag. 2007, 48, 1499–1505. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-Efficient Polymeric Gas Separation Membranes for a Sustainable Future: A Review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Chen, X.Y.; Kaliaguine, S.; Rodrigue, D. A Comparison between Several Commercial Polymer Hollow Fiber Membranes for Gas Separation. AIP Conf. Proc. 2019, 2139, 070003. [Google Scholar] [CrossRef]
- David, O.C.; Gorri, D.; Nijmeijer, K.; Ortiz, I.; Urtiaga, A. Hydrogen Separation from Multicomponent Gas Mixtures Containing CO, N2 and CO2 Using Matrimid® Asymmetric Hollow Fiber Membranes. J. Memb. Sci. 2012, 419–420, 49–56. [Google Scholar] [CrossRef]
- Clausi, D.T.; Koros, W.J. Formation of Defect-Free Polyimide Hollow Fiber Membranes for Gas Separations. J. Memb. Sci. 2000, 167, 79–89. [Google Scholar] [CrossRef]
- Ayub, M.; Othman, M.H.D.; Kadir, S.H.S.A.; Ali, A.; Khan, I.U.; Yusop, M.Z.M.; Matsuura, T.; Fauzi Ismail, A.; Rahman, M.A.; Jaafar, J. Research and Development Journey and Future Trends of Hollow Fiber Membranes for Purification Applications (1970–2020): A Bibliometric Analysis. Membranes 2021, 11, 600. [Google Scholar] [CrossRef]
- David, O.C.; Gorri, D.; Urtiaga, A.; Ortiz, I. Mixed Gas Separation Study for the Hydrogen Recovery from H2/CO/N2/CO2 Post Combustion Mixtures Using a Matrimid Membrane. J. Memb. Sci. 2011, 378, 359–368. [Google Scholar] [CrossRef]
- Rao, H.X.; Liu, F.N.; Zhang, Z.Y. Preparation and Oxygen/Nitrogen Permeability of PDMS Crosslinked Membrane and PDMS/Tetraethoxysilicone Hybrid Membrane. J. Memb. Sci. 2007, 303, 132–139. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Martin-Gil, V.; Ahmad, M.Z.; Fíla, V. Matrimid® 5218 in Preparation of Membranes for Gas Separation: Current State-of-the-Art. Chem. Eng. Commun. 2018, 205, 161–196. [Google Scholar] [CrossRef]
- Fernández-Castro, P.; Ortiz, A.; Gorri, D. Exploring the Potential Application of Matrimid® and ZIFs-Based Membranes for Hydrogen Recovery: A Review. Polymers 2021, 13, 1292. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V. Progress on Incorporating Zeolites in Matrimid(®)5218 Mixed Matrix Membranes towards Gas Separation. Membranes 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, E.; Minelli, M.; De Angelis, M.G. Modelling Sorption and Transport of Gases in Polymeric Membranes across Different Scales: A Review. Membranes 2022, 12, 857. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ansaloni, L.; Deng, L. Recent Advances in Multi-Layer Composite Polymeric Membranes for CO2 Separation: A Review. Green Energy Environ. 2016, 1, 102–128. [Google Scholar] [CrossRef]
- Li, G.; Kujawski, W.; Válek, R.; Koter, S. A Review—The Development of Hollow Fibre Membranes for Gas Separation Processes. Int. J. Greenh. Gas Control. 2021, 104, 103195. [Google Scholar] [CrossRef]
- Arregoitia-Sarabia, C.; González-Revuelta, D.; Fallanza, M.; Ortiz, A.; Gorri, D. PEBA/PDMS Composite Multilayer Hollow Fiber Membranes for the Selective Separation of Butanol by Pervaporation. Membranes 2022, 12, 1007. [Google Scholar] [CrossRef]
- Chong, K.C.; Lai, S.O.; Lau, W.J.; Thiam, H.S.; Ismail, A.F.; Roslan, R.A. Preparation, Characterization, and Performance Evaluation of Polysulfone Hollow Fiber Membrane with PEBAX or PDMS Coating for Oxygen Enhancement Process. Polymers 2018, 10, 126. [Google Scholar] [CrossRef]
- Chen, S.H.; Wu, T.H.; Ruaan, R.C.; Lai, J.Y. Effect of Top Layer Swelling on the Oxygen/Nitrogen Separation by Surface Modified Polyurethane Membranes. J. Memb. Sci. 1998, 141, 255–264. [Google Scholar] [CrossRef]
- Simons, K.; Nijmeijer, K.; Sala, J.G.; van der Werf, H.; Benes, N.E.; Dingemans, T.J.; Wessling, M. CO2 Sorption and Transport Behavior of ODPA-Based Polyetherimide Polymer Films. Polymer 2010, 51, 3907–3917. [Google Scholar] [CrossRef]
- Widjojo, N.; Chung, T.-S. The Thickness and Air Gap Dependence of Macrovoid Evolution in Phase-Inversion Asymmetric Hollow Fiber Membranes. In Hollow Fiber Membranes; Chung, T.-S., Feng, Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume 45, pp. 123–140. ISBN 9780128218761. [Google Scholar]
- Le, N.L.; Pulido, B.A.; Nunes, S.P. Fabrication of Hollow Fiber Membranes Using Highly Viscous Liquids as Internal Coagulants. Ind. Eng. Chem. Res. 2019, 58, 22343–22349. [Google Scholar] [CrossRef]
- Wang, Y.; Goh, S.H.; Chung, T.S.; Na, P. Polyamide-Imide/Polyetherimide Dual-Layer Hollow Fiber Membranes for Pervaporation Dehydration of C1-C4 Alcohols. J. Memb. Sci. 2009, 326, 222–233. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Li, K. Effect of Ethanol Composition in Water Coagulation Bath on Morphology of PVDF Hollow Fibre Membranes. J. Memb. Sci. 1998, 150, 75–85. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Otitoju, T.A.; Ooi, B.S. Hollow Fiber (HF) Membrane Fabrication: A Review on the Effects of Solution Spinning Conditions on Morphology and Performance. J. Ind. Eng. Chem. 2019, 70, 35–50. [Google Scholar] [CrossRef]
- Himma, N.F.; Wenten, I.G. Surface Engineering of Polymer Membrane for Air Separation. AIP Conf. Proc. 2017, 1840, 090005. [Google Scholar] [CrossRef]
- Arregoitia-Sarabia, C.; González-Revuelta, D.; Fallanza, M.; Ortiz, A.; Gorri, D. Polyether-Block-Amide Thin-Film Composite Hollow Fiber Membranes for the Recovery of Butanol from ABE Process by Pervaporation. Sep. Purif. Technol. 2021, 279, 119758. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, J.; Li, Y.; Liu, J.; Zhou, J.; Cen, K. Optimization of Coating Solution Viscosity of Hollow Fiber-Supported Polydimethylsiloxane Membrane for CO2/H2 Separation. J. Appl. Polym. Sci. 2018, 135, 45765. [Google Scholar] [CrossRef]
- Li, G.; Kujawski, W.; Knozowska, K.; Kujawa, J. The Effects of PEI Hollow Fiber Substrate Characteristics on PDMS/PEI Hollow Fiber Membranes for CO2/N2 Separation. Membranes 2021, 11, 56. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Hughes, R. Preparation of Porous Aluminium Oxide (Al2O3) Hollow Fibre Membranes by a Combined Phase-Inversion and Sintering Method. Ceram. Int. 2003, 29, 875–881. [Google Scholar] [CrossRef]
- Kingsbury, B.F.K.; Li, K. A Morphological Study of Ceramic Hollow Fibre Membranes. J. Memb. Sci. 2009, 328, 134–140. [Google Scholar] [CrossRef]
- Guiver, M.D.; Robertson, G.P.; Dai, Y.; Bilodeau, F.; Kang, Y.S.; Lee, K.J.; Jho, J.Y.; Won, J. Structural Characterization and Gas-Transport Properties of Brominated Matrimid Polyimide. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4193–4204. [Google Scholar] [CrossRef]
- Xiao, Y.; Dai, Y.; Chung, T.S.; Guiver, M.D. Effects of Brominating Matrimid Polyimide on the Physical and Gas Transport Properties of Derived Carbon Membranes. Macromolecules 2005, 38, 10042–10049. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kapantaidakis, G.C.; Nolan, J.W.; Mitropoulos, A.C.; Kanellopoulos, N.K. Preparation, Characterization and Gas Permeation Properties of Carbon Hollow Fiber Membranes Based on Matrimid® 5218 Precursor. J. Mater. Process. Technol. 2007, 186, 102–110. [Google Scholar] [CrossRef]
- Ding, X.; Cao, Y.; Zhao, H.; Wang, L.; Yuan, Q. Fabrication of High Performance Matrimid/Polysulfone Dual-Layer Hollow Fiber Membranes for O2/N2 Separation. J. Memb. Sci. 2008, 323, 352–361. [Google Scholar] [CrossRef]
- Pian, C.; Shen, J.; Liu, G.; Liu, Z.; Jin, W. Ceramic Hollow Fiber-Supported PDMS Composite Membranes for Oxygen Enrichment from Air. ASIA-PACIFIC J. Chem. Eng. 2016, 11, 460–466. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Shahidi, K.; Mohammadi, T. Synthesis and Gas Permeation Properties of a Single Layer PDMS Membrane. J. Appl. Polym. Sci. 2010, 117, 33–48. [Google Scholar] [CrossRef]
- Koros, W.J.; Woods, D.G. Elevated Temperature Application of Polymer Hollow-Fiber Membranes. J. Memb. Sci. 2001, 181, 157–166. [Google Scholar] [CrossRef]
- Barsema, J.N.; Kapantaidakis, G.C.; Van Der Vegt, N.F.A.; Koops, G.H.; Wessling, M. Preparation and Characterization of Highly Selective Dense and Hollow Fiber Asymmetric Membranes Based on BTDA-TDI/MDI Co-Polyimide. J. Memb. Sci. 2003, 216, 195–205. [Google Scholar] [CrossRef]
- Choi, S.H.; Jansen, J.C.; Tasselli, F.; Barbieri, G.; Drioli, E. In-Line Formation of Chemically Cross-Linked P84® Co-Polyimide Hollow Fibre Membranes for H2/CO2 Separation. Sep. Purif. Technol. 2010, 76, 132–139. [Google Scholar] [CrossRef]
- Thornton, A.W.; Freeman, B.D.; Robeson, L.M. Polymer Gas Separation Membrane Database. Available online: https://membrane-australasia.org/polymer-gas-separation-membrane-database/ (accessed on 25 July 2022).
- Burdyny, T.; Struchtrup, H. Hybrid Membrane/Cryogenic Separation of Oxygen from Air for Use in the Oxy-Fuel Process. Energy 2010, 35, 1884–1897. [Google Scholar] [CrossRef]
- Bisio, G.; Bosio, A.; Rubatto, G. Thermodynamics Applied to Oxygen Enrichment of Combustion Air. Energy Convers. Manag. 2002, 43, 2589–2600. [Google Scholar] [CrossRef]














| HF Membrane | PVDF | Matrimid |
|---|---|---|
| Dope composition (wt.%) | 16 | 20 |
| Solvent | NMP | NMP |
| Additive content (wt %) | 1 | - |
| Dope flow rate (mL min−1) | 2 | 3 |
| Dope extrusion temperature (°C) | 20 | 60 |
| Bore liquid | Ethanol 20 wt.% (Milli-Q water) | NMP 20 wt.% (Milli-Q water) |
| Bore liquid temperature (°C) | 20 | 20 |
| Bore liquid flow rate (mL min−1) | 1 | 3 |
| External coagulant | Water | Water |
| External coagulant temperature (°C) | 30 | 15 |
| Air gap (cm) | 5 | 6 |
| Take up speed (m min−1) | 12 | 12 |
| Coagulation bath depth (m) | 1.5 | 1.5 |
| Inner diameter (μm) | 420 | 580 |
| Wall thickness (µm) | 170 | 50 |
| Feed Flow (mL/min) | Sweep Gas Flow (mL/min) | Total Feed Pressure (bar) | Total Permeate Pressure (bar) | |
|---|---|---|---|---|
| Matrimid | 180–200 | 5–5.2 | 1.2–4.5 | 1 |
| PDMS | 180–220 | 15–30 | 1.2–4.5 | 1 |
| Matrimid + PDMS | 180–220 | 5–5.8 | 1.2–4.5 | 1 |
| T (°C) | Pure Gases Feed | Mixture 50:50 Feed | ||||
|---|---|---|---|---|---|---|
| P O2 (GPU) | P N2 (GPU) | α (-) ideal | P O2 (GPU) | P N2 (GPU) | α (-) | |
| 20 | - | - | - | 15.4 | 11.8 | 1.3 |
| 30 | 13.6 | 8.4 | 1.6 | 20.5 | 14.7 | 1.4 |
| 40 | 17.7 | 11.5 | 1.5 | 26.9 | 18.3 | 1.5 |
| 50 | 22.8 | 15.3 | 1.5 | 31.2 | 19.8 | 1.6 |
| T (°C) | P O2 (GPU) | P N2 (GPU) | α (-) |
|---|---|---|---|
| 30 | 3.8 | 2.1 | 1.8 |
| 40 | 4.5 | 2.5 | 1.8 |
| 50 | 5.3 | 3.4 | 1.6 |
| T (°C) | Pure Gases Feed | Mixture 50:50 Feed | ||||
|---|---|---|---|---|---|---|
| P O2 (GPU) | P N2 (GPU) | α (-) Ideal | P O2 (GPU) | P N2 (GPU) | α (-) | |
| 30 | 3.6 | 0.7 | 4.8 | 4.5 | 1 | 4.6 |
| 40 | 5.7 | 1.1 | 5.3 | 6.9 | 1.2 | 5.6 |
| 50 | 8.4 | 1.6 | 5.3 | 11.7 | 2.2 | 5.3 |
| 60 | 11.6 | 2.6 | 4.5 | 19.4 | 3.9 | 5 |
| 70 | - | - | - | 22.6 | 4.8 | 4.7 |
| 80 | - | - | - | 30.8 | 6.6 | 4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Revuelta, D.; Fallanza, M.; Ortiz, A.; Gorri, D. Thin-Film Composite Matrimid-Based Hollow Fiber Membranes for Oxygen/Nitrogen Separation by Gas Permeation. Membranes 2023, 13, 218. https://doi.org/10.3390/membranes13020218
González-Revuelta D, Fallanza M, Ortiz A, Gorri D. Thin-Film Composite Matrimid-Based Hollow Fiber Membranes for Oxygen/Nitrogen Separation by Gas Permeation. Membranes. 2023; 13(2):218. https://doi.org/10.3390/membranes13020218
Chicago/Turabian StyleGonzález-Revuelta, Daniel, Marcos Fallanza, Alfredo Ortiz, and Daniel Gorri. 2023. "Thin-Film Composite Matrimid-Based Hollow Fiber Membranes for Oxygen/Nitrogen Separation by Gas Permeation" Membranes 13, no. 2: 218. https://doi.org/10.3390/membranes13020218