Study on UF PES Membranes Spray-Coated with Polymerizable Bicontinuous Microemulsion Materials for Low-Fouling Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. PBM Preparation
2.2. Commercial Membrane as Reference
2.3. Low-Fouling Layer Coating
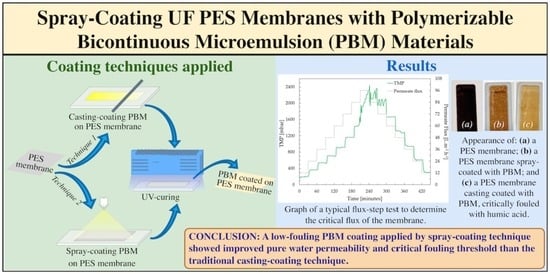
2.3.1. Casting-Coating Technique
2.3.2. Spray-Coating Technique
2.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5. Contact Angle Measurement (CAM)
2.6. Scanning Electron Microscopy (SEM)
2.7. Confocal Microscopy
2.8. Membrane Cross-Flow Test Unit
2.9. Critical Flux Determination
3. Results and Discussion
3.1. Membrane Morphology and Properties
3.1.1. ATR-FTIR Spectroscopy
3.1.2. SEM Imaging
3.1.3. Determination of Thickness of Coated PBM Layer Using Confocal Microscope
3.1.4. Water Contact Angle (CA)
3.2. Water Permeability and Rejection Tests
3.2.1. Pure Water Permeability (PWP)
3.2.2. Low-Fouling Tests
3.2.3. Rejection Rate
3.3. Characterisation of the Membranes Fouled with HA
3.3.1. ATR-FTIR Spectroscopy of PES Membranes Fouled with HA
3.3.2. SEM Images of PES Membranes Fouled with HA
3.3.3. CAM of PES Membranes Fouled with HA
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fane, A.G.; Tang, C.Y.; Wang, R. Membrane technology for water: Microfiltration, ultrafiltration, nanofiltration, and reverse Osmosis. In Treatise on Water Science; Wilderer, P., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2011; Volume 4, pp. 301–335. ISBN 978-0-444-53199-5. [Google Scholar]
- Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors in Water and Wastewater Treatment; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; ISBN 978-1-85-617481-7. [Google Scholar]
- Noble, R.D.; Stern, S.A. Membrane Separations Technology—Principles and Applications, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 1995; ISBN 9780080536187. [Google Scholar]
- Díez, B.; Rosal, R. A critical review of membrane modification techniques for fouling and biofouling control in pressure-driven membrane processes. Nanotechnol. Environ. Eng. 2020, 5, 15. [Google Scholar] [CrossRef]
- Poosagari, W.; Bugge, T.V.; Christensen, M.L.; Jørgensen, M.K. Compressibility of fouling layers in membrane bioreactors. J. Membr. Sci. 2015, 475, 65–70. [Google Scholar]
- Le Clech, P.; Chen, V.; Fane, T.A.G. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Pollice, A.; Brookes, A.; Jefferson, B.; Judd, S. Sub-critical flux fouling in membrane bioreactors—A review of recent literature. Desalination 2005, 174, 221–230. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, X.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar]
- Gabarrón, S.; Ferrero, G.; Dalmau, M.; Comas, J.; Rodriguez-Roda, I. Assessment of energy-saving strategies and operational costs in full-scale membrane bioreactors. J. Environ. Manag. 2014, 134, 8–14. [Google Scholar] [CrossRef]
- Monclús, H.; Dalmau, M.; Gabarrón, S.; Ferrero, G.; Rodríguez-Roda, I.; Comas, J. Full-scale validation of an air scour control system for energy savings in membrane bioreactors. Water Res. 2015, 79, 1–9. [Google Scholar] [CrossRef]
- Gao, Y.; Qin, J.; Wang, Z.; Østerhus, S.W. Backpulsing technology applied in MF and UF processes for membrane fouling mitigation: A review. J. Membr. Sci. 2019, 587, 117–136. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons, Ltd.: London, UK, 2004; ISBN 0470854456. [Google Scholar]
- Susanto, H.; Ulbricht, M. Characteristics, performance and stability of polyethersulfone ultrafiltration membranes prepared by phase separation method using different macromolecular additives. J. Membr. Sci. 2009, 327, 125–135. [Google Scholar] [CrossRef]
- Al-Shaeli, M.; Hegab, H.M.; Fang, X.; He, L.; Liu, C.; Wang, H.; Zhang, K.; Ladewig, B.P. Reduced fouling ultrafiltration membranes via in-situ polymerisation using polydopamine functionalised titanium oxide. In ChemRxiv; Cambridge Open Engage: Cambridge, UK, 2020. [Google Scholar]
- Nady, N. PES Surface modification using green chemistry: New generation of antifouling membranes. Membranes 2016, 6, 23. [Google Scholar] [CrossRef]
- Lou, D.; Hou, Z.; Yang, H.; Liu, Y.; Wang, T. Antifouling membranes prepared from polyethersulfone grafted with poly(ethylene glycol) methacrylate by radiation-induced copolymerization in homogeneous solution. ACS Omega 2020, 5, 27094–27102. [Google Scholar] [CrossRef] [PubMed]
- Al Hachim, Z.S.; Ridha, A.M.; Al-Baiati, M.N.; Alsalhy, Q.F.; Majdi, H.S. Sustainable modification of polyethersulfone membrane with poly(maleic anhydride-co-glycerol) as novel copolymer. Water 2022, 14, 1207. [Google Scholar] [CrossRef]
- Wang, M.; Sun, F.; Zeng, H.; Su, X.; Zhou, G.; Liu, H.; Xing, D. Modified polyethersulfone ultrafiltration membrane for enhanced antifouling capacity and dye catalytic degradation efficiency. Separations 2022, 9, 92. [Google Scholar] [CrossRef]
- Galiano, F. Preparation and Characterization of Polymersiable Bicontinuous Microemulsion Membranes for Water Treatment Application. Ph.D. Thesis, University of Calabria, Cosenza, Italy, 2013. Available online: http://hdl.handle.net/10955/995 (accessed on 27 August 2023).
- Galiano, F.; Figoli, A.; Deowan, S.A.; Johnson, D.; Altinkaya, S.A.; Veltri, L.; De Luca, G.; Mancuso, R.; Hilal, N.; Gabriele, B. A step forward to a more efficient wastewater treatment by membrane surface modification via polymerizable bicontinuous microemulsion. J. Membr. Sci. 2015, 482, 103–114. [Google Scholar] [CrossRef]
- Deowan, S.A.; Galiano, F.; Hoinkis, J.; Johnson, D.; Altinkaya, S.A.; Gabriele, B.; Hilal, N.; Drioli, E.; Figoli, A. Novel low-fouling membrane bioreactor for industrial wastewater treatment. J. Membr. Sci. 2016, 510, 524–532. [Google Scholar] [CrossRef]
- Gukelberger, E.; Hitzel, C.; Mancuso, R.; Galiano, F.; Bruno, M.D.L.; Simonutti, R.; Gabriele, B.; Figoli, A.; Hoinkis, J. Viscosity modification of polymerizable bicontinuous microemulsion by controlled radical polymerization for membrane coating applications. Membranes 2020, 10, 246. [Google Scholar] [CrossRef]
- Galiano, F.; Schmidt, S.A.; Ye, X.; Kumar, R.; Mancuso, R.; Curcio, E.; Gabriele, B.; Hoinkis, J.; Figoli, A. UV-LED induced bicontinuous microemulsions polymerisation for surface modification of commercial membranes—Enhancing the antifouling properties. Sep. Purif. Technol. 2018, 194, 149–160. [Google Scholar] [CrossRef]
- Klingele, M.; Britton, B.; Breitwieser, M.; Vierrath, S.; Zengerle, R.; Holdcroft, S.; Thiele, S. A completely spray-coated membrane electrode assembly. Electrochem. Commun. 2016, 70, 65–68. [Google Scholar] [CrossRef]
- Sparks, B.J.; Hoff, E.F.T.; Xiong, L.; Goetz, J.T.; Patton, D.L. Superhydrophobic hybrid inorganic–Organic thiol-ene surfaces fabricated via spray-deposition and photopolymerization. Appl. Mater. Interfaces 2013, 5, 1811–1817. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Wu, L.; Wang, A. Durable superhydrophobic surfaces prepared by spray coating of polymerised organosilane/attapulgite nanocomposites. ChemPlusChem 2013, 78, 1503–1509. [Google Scholar] [CrossRef]
- Wozniak, G. Tropfenbildungsmechanismen. In Zerstäubungstechnik: Prinzipien, Verfahren, Geräte; Springer: Berlin/Heidelberg, Germany, 2003; pp. 31–50. ISBN 978-3-540-41170-3. [Google Scholar]
- Mancuso, R.; Amuso, R.; Armentano, B.; Grasso, G.; Rago, V.; Capello, A.R.; Galiano, F.; Figoli, A.; De Luca, G.; Hoinkis, J.; et al. Synthesis and antimicrobial activity of polymerisable acryloyloxyalkyltriethyl ammonium salts. ChemPlusChem 2017, 82, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Figoli, A.; Hoinkis, J.; Gabriele, B.; De Luca, G.; Galiano, F.; Deowan, S.A. Bicontinuous Microemulsion Polymerised Coating for Water Treatment. European Patent EP3049178, 8 April 2020. [Google Scholar]
- DIN EN ISO 14175:N1; Product Data Sheet: Nitrogen. Linde GmbH, Gases Division: Pullach, Germany, 2016.
- Mann+Hummel, Product Specification–NADIR® UP150 P Ultrafiltration Membrane. Available online: https://water-fluid-filtration.mann-hummel.com/content/dam/lse-wfs/product-related-assets/data-sheets/UP150-P-Flat-Sheet-Membrane.pdf/_jcr_content/renditions/original./UP150-P-Flat-Sheet-Membrane.pdf (accessed on 7 January 2021).
- Mann+Hummel, Product Specification–BIO-CEL® L-2 Submerged MBR Module for Wastewater Treatment, Membrane Characteristics. Available online: https://water-fluid-filtration.mann-hummel.com/content/dam/lse-wfs/communication-media/brochures-catalogs/MICRODYN-BIO-CEL-L-2-MBR-Module-Brochure.pdf/_jcr_content/renditions/original./MICRODYN-BIO-CEL-L-2-MBR-Module-Brochure.pdf (accessed on 8 October 2021).
- Wicks, Z.W., Jr.; Jones, F.N.; Pappas, S.P.; Douglas, A.; Wicks, D.A. Chapter 2: Polymerization and Film Formation. In Organic Coatings: Science and Technology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-471-69806-7. [Google Scholar]
- Greisinger. Operating Manual–OXY3690 MP: Measuring Transducer for Air Oxygen. GHM Messtechnik GmbH. Available online: www.greisinger.de (accessed on 8 January 2021).
- TQC Sheen. Datasheet–Spiral Bar Coated AB3050. Available online: www.industrialphysics.com (accessed on 10 December 2022).
- Lechler GmbH. Product Specification–Series 176 ViscoMist. Available online: www.eshop.lechler.de (accessed on 7 July 2022).
- Olympus. Datasheet–3D Measuring Laser Microscope Lext OLS4100. Available online: www.olympus-global.com (accessed on 8 July 2022).
- SIMA-tec. Datasheet–Laboratory Membrane Test Unit LSta05. Available online: www.sima-tec-gmbh.de (accessed on 17 May 2023).
- Clech, P.L.; Jefferson, B.; Chang, I.S.; Judd, S.J. Critical flux determination by the flux-step method in a submerged membrane bioreactor. J. Membr. Sci. 2003, 227, 81–93. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S. Improvement of performance and surface properties of nano-porous polyethersulfone (PES) membrane using hydrophilic monomers as additives in the casting solution. J. Membr. Sci. 2010, 360, 371–379. [Google Scholar] [CrossRef]
- Guzelot, C.; Lahitte, J.F.; Remigy, J.C. Dip coatng of microemulsions on hollow fibres for improved membrane filtration performance: An industrial perspective? MATEC Web Conf. 2023, 379, 08002. [Google Scholar] [CrossRef]
- Deowan, S.A. Development of Membrane Bioreactor (MBR) Process Applying Novel Low Fouling Membranes. Ph.D. Thesis, University of Calabria, Cosenza, Italy, 2013. Available online: http://hdl.handle.net/10955/772 (accessed on 27 August 2023). [CrossRef]
- Cameron, R.S.; Thornton, B.K.; Swift, R.S.; Posner, A.M. Molecular weight and shape of humic acid from sedimentation and diffusion measurements on fractionated extracts. Eur. J. Soil Sci. 2006, 23, 394–408. [Google Scholar] [CrossRef]
- Yang, F.; Antonietti, M. The sleeping giant: A polymer view on humic matter in synthesis and applications. Prog. Polym. Sci. 2019, 100, 101182. [Google Scholar] [CrossRef]
- Platkowska-Siwied, A.; Bodzek, M. Influence of natural organic matter on fouling and ultrafiltration membranes properties—AFM analysis. Ecol. Chem. Eng. A 2012, 19, 1561–1570. [Google Scholar] [CrossRef]











| Membrane Modification Approach | Improved Membrane Performance | Ref. |
|---|---|---|
| Addition of polyvinylpyrrolidone (PVP), polyethylene glycol (PEG) and (Pluronic®, Plu) to the PES membrane prepared via a non-solvent-induced phase separation (NIPS) method. | Compaction study (450 kPa for 2 h) showed PES membrane had highest pure water flux (PWF) (~120 L m−2 h−1) followed by PES-Plu (~70 L m−2 h−1), PES-PEG (~55 L m−2 h−1), and PES-PVP (~40 L m−2 h−1). UF experiments of the anti-fouling membranes with 0.1 g L−1 bovine serum albumin (BSA) at 300 kPa showed that PES-Plu had the highest permeate flux and highest protein rejection rate. | [13] |
| Binding titanium dioxide (TiO2) nanoparticles using dopamine (DA) adhesive on PES membrane surface via dip-coating. | Hybrid DA-TiO2 PES membrane showed pure water flux (PWF) improvement from 79.9 L m−2 h−1 to 962 L m−2 h−1. A fouling test with 1 g L−1 BSA at 0.1 MPa showed rejection improvement in rejection rate of 10–20%. | [14] |
| Graft polymeric oligomers using ferulic acid modifier and laccase bio-catalyst on the PES membrane. | The PWF of modified membranes reduced by an average of 10%. Antifouling property was improved due to 94% reduction in protein adsorption (BSA). | [15] |
| Cast membranes using PES/PEG blend and graft PEG methacrylate on PES (PES-g-polyPEGMA) via an NIPS method. | The PWF of PES-g-polyPEGMA was higher (~170 L m−2 h−1) than the PES/PEG membrane. Fouling tests with BSA (1 g L−1) at 0.1 MPa and surface velocity of 2 kg h−1 showed higher rejection rate (~90%) than PES-g-PEGMA membrane (~98%). The latter was highlighted due to its improved anti-fouling properties. | [16] |
| Cast PES membranes using nanocomposite graft copolymer additive—(poly(maleic anhydride-co-glycerol), PMG) nanoparticles via an NIPS method | PMG-PES membranes had highest PWF (~908 L m−2 h−1) than PES membranes (~150 L m−2 h−1). Fouling tests using BSA at 1 bar showed a 98% rejection rate for the PMG-PES membrane as compared to 69% for the PES membranes. | [17] |
| Cast composite membrane using molybdenum disulfide-iron oxyhydroxide, MoS2-FeOOH/PES via a phase inversion method. | PWF of MoS2-FeOOH/PES membranes was 1.7 times higher (~385.3 L m−2 h−1) than PES membranes (~225 L m−2 h−1). Fouling tests using BSA (0.5 g L−1) at 0.1 MPa showed a rejection rate of 91% for the anti-fouling composite membranes and 96% for the PES membranes. | [18] |
| Coating Substrate | PBM Coating Technique | Thickness of Coated PBM Layer |
|---|---|---|
| PES membrane surface | Casting-coated | 4.85 ± 1.00 µm |
| PES membrane surface | Spray-coated | Could not be determined 1 |
| Alumina surface | Casting-coated | 4.60 ± 0.50 µm |
| Alumina surface | Spray-coated | 1.70 ± 0.10 µm |
| Membrane Surface | CAM (Average) | Reduction in CAM |
|---|---|---|
| PES | 73° ± 3° | - |
| Casting-coated PBM on PES | 65° ± 3° | Reduced by 11.0% from PES |
| Spray-coated PBM on PES | 66° ± 2° | Reduced by 9.6% from PES |
| Membrane Sample | Rejection Rate | Improvement in Rejection Rate |
|---|---|---|
| Commercial PES | 33% | - |
| PES casting-coated with PBM | 55% | Increased by 22% from PES |
| PES spray-coated with PBM | 50% | Increased by 17% from PES |
| Membrane Surface | CAM (Average) | Change in CA after HA Fouling |
|---|---|---|
| PES | 86° ± 1° | Increased |
| Casting-coated PBM on PES | 57° ± 1° | Decreased |
| Spray-coated PBM on PES | 50° ± 2° | Decreased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De, S.; Heer, J.; Sankar, S.; Geiger, F.; Gukelberger, E.; Galiano, F.; Mancuso, R.; Gabriele, B.; Figoli, A.; Hoinkis, J. Study on UF PES Membranes Spray-Coated with Polymerizable Bicontinuous Microemulsion Materials for Low-Fouling Behavior. Membranes 2023, 13, 893. https://doi.org/10.3390/membranes13120893
De S, Heer J, Sankar S, Geiger F, Gukelberger E, Galiano F, Mancuso R, Gabriele B, Figoli A, Hoinkis J. Study on UF PES Membranes Spray-Coated with Polymerizable Bicontinuous Microemulsion Materials for Low-Fouling Behavior. Membranes. 2023; 13(12):893. https://doi.org/10.3390/membranes13120893
Chicago/Turabian StyleDe, Sneha, Jonathan Heer, Suwetha Sankar, Fabian Geiger, Ephraim Gukelberger, Francesco Galiano, Raffaella Mancuso, Bartolo Gabriele, Alberto Figoli, and Jan Hoinkis. 2023. "Study on UF PES Membranes Spray-Coated with Polymerizable Bicontinuous Microemulsion Materials for Low-Fouling Behavior" Membranes 13, no. 12: 893. https://doi.org/10.3390/membranes13120893