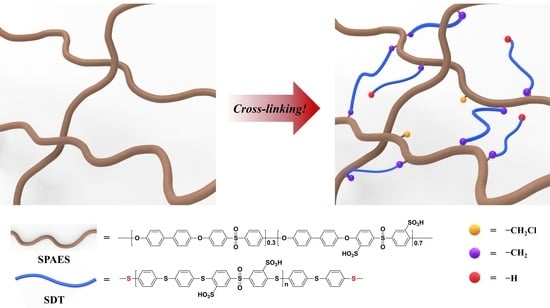
Cross-Linked Sulfonated Poly(arylene ether sulfone) Membrane Using Polymeric Cross-Linkers for Polymer Electrolyte Membrane Fuel Cell Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Sulfonated Poly(arylene ether sulfone) (SPAES)
2.3. Modification of SPAES to SPAES with Chloromethyl Moieties (SPAES-Cl)
2.4. Synthesis of Thiophenoxide-Terminated Polymeric Cross-Linkers
2.5. Preparation of Cross-Linked SPAES Membrane
2.6. Preparation of Membrane Electrode Assemblies (MEAs)
2.7. Characterization
3. Results and Discussion
3.1. Synthesis and Modification of SPAES and Polymeric Cross-Linker
3.2. Preparation of Cross-Linked SPAES Membranes
3.3. Swelling Ratio, Water Uptake, and Mechanical Properties of the Cross-Linked Membranes
3.4. Proton Conductivity and Cell Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, C.; Zhang, H. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel Cell and Electrolysis Cell Technologies and Hydrogen Infrastructure Development: A Review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Liu, H.; Gong, C.; Wen, S.; Wang, X.; Tu, Z. Progress on design and development of polymer electrolyte membrane fuel cell systems for vehicle applications: A review. Fuel Process. Technol. 2018, 179, 203–228. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.-F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, H.; Jeong, Y.-G.; Choi, C.; Hwang, I.; Song, S.; Nam, S.Y.; Lee, J.H.; Kim, K. Polymer Electrolyte Membranes Containing Functionalized Organic/Inorganic Composite for Polymer Electrolyte Membrane Fuel Cell Applications. Int. J. Mol. Sci. 2022, 23, 14252. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Belgacem, I.B.; Emori, W.; Uzoma, P.C. Nafion degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrogen Energy 2021, 46, 27956–27973. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzu, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Rosli, R.; Sulong, A.; Daud, W.; Zulkifley, M.; Husaini, T.; Rosli, M.; Majlan, E.; Haque, M. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.H.; Guiver, M.D.; Lee, Y.M. Sulfonated hydrocarbon membranes for medium-temperature and low-humidity proton exchange membrane fuel cells (PEMFCs). Prog. Polym. Sci. 2011, 36, 1443–1498. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Heo, P.; Han, J.; Kim, J.; Lee, J.-C. End-group cross-linked sulfonated poly (arylene ether sulfone) via thiol-ene click reaction for high-performance proton exchange membrane. J. Power Sources 2018, 401, 20–28. [Google Scholar] [CrossRef]
- Yuk, J.; Lee, S.; Nugraha, A.F.; Lee, H.; Park, S.-H.; Yim, S.-D.; Bae, B. Synthesis and characterization of multi-block poly (arylene ether sulfone) membranes with highly sulfonated blocks for use in polymer electrolyte membrane fuel cells. J. Membr. Sci. 2016, 518, 50–59. [Google Scholar] [CrossRef]
- Lade, H.; Kumar, V.; Arthanareeswaran, G.; Ismail, A. Sulfonated poly (arylene ether sulfone) nanocomposite electrolyte membrane for fuel cell applications: A review. Int. J. Hydrogen Energy 2017, 42, 1063–1074. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, J.; Han, J.; Kim, K.; Park, S.; Kim, S.; Park, H.S.; Cho, Y.-H.; Lee, J.-C.; Sung, Y.-E. High-performance proton-exchange membrane water electrolysis using a sulfonated poly (arylene ether sulfone) membrane and ionomer. J. Membr. Sci. 2021, 620, 118871. [Google Scholar] [CrossRef]
- Harun, N.A.M.; Shaari, N.; Nik Zaiman, N.F.H. A review of alternative polymer electrolyte membrane for fuel cell application based on sulfonated poly (ether ether ketone). Int. J. Energy Res. 2021, 45, 19671–19708. [Google Scholar] [CrossRef]
- Kim, J.; Han, J.; Kim, H.; Kim, K.; Lee, H.; Kim, E.; Choi, W.; Lee, J.-C. Thermally cross-linked sulfonated poly (ether ether ketone) membranes containing a basic polymer-grafted graphene oxide for vanadium redox flow battery application. J. Energy Storage 2022, 45, 103784. [Google Scholar] [CrossRef]
- Thong, P.T.; Sadhasivam, T.; Lim, H.; Jin, C.-S.; Ryi, S.-K.; Park, W.; Kim, H.T.; Roh, S.-H.; Jung, H.-Y. High oxidizing stability and ion selectivity of hybrid polymer electrolyte membrane for improving electrochemical performance in vanadium redox flow battery. J. Electrochem. Soc. 2018, 165, A2321. [Google Scholar] [CrossRef]
- Yadav, V.; Niluroutu, N.; Bhat, S.D.; Kulshrestha, V. Insight toward the electrochemical properties of sulfonated poly (2, 6-dimethyl-1, 4-phenylene oxide) via impregnating functionalized boron nitride: Alternate composite polymer electrolyte for direct methanol fuel cell. ACS Appl. Energy Mater. 2020, 3, 7091–7102. [Google Scholar] [CrossRef]
- Kim, M.; Ko, H.; Nam, S.Y.; Kim, K. Study on control of polymeric architecture of sulfonated hydrocarbon-based polymers for high-performance polymer electrolyte membranes in fuel cell applications. Polymers 2021, 13, 3520. [Google Scholar] [CrossRef]
- Han, J.; Lee, H.; Kim, J.; Kim, S.; Kim, H.; Kim, E.; Sung, Y.-E.; Kim, K.; Lee, J.-C. Sulfonated poly (arylene ether sulfone) composite membrane having sulfonated polytriazole grafted graphene oxide for high-performance proton exchange membrane fuel cells. J. Membr. Sci. 2020, 612, 118428. [Google Scholar] [CrossRef]
- Al Munsur, A.Z.; Goo, B.-H.; Kim, Y.; Kwon, O.J.; Paek, S.Y.; Lee, S.Y.; Kim, H.-J.; Kim, T.-H. Nafion-based proton-exchange membranes built on cross-linked semi-interpenetrating polymer networks between poly (acrylic acid) and poly (vinyl alcohol). ACS Appl. Mater. Interfaces 2021, 13, 28188–28200. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, X.; Qian, H.; Xu, J.; Yue, Z.; Zou, Z.; Yang, H. Cross-linked sulfonated poly (ether ether ketone) electrolytes bearing pendent imidazole groups for high temperature proton exchange membrane fuel cells. Sustain. Energy Fuels 2019, 3, 2426–2434. [Google Scholar] [CrossRef]
- Hu, E.N.; Lin, C.X.; Liu, F.H.; Yang, Q.; Li, L.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Cross-linked poly (vinylbenzyl chloride) anion exchange membranes with long flexible multihead for fuel cells. ACS Appl. Energy Mater. 2018, 1, 3479–3487. [Google Scholar] [CrossRef]
- Kim, K.; Heo, P.; Hwang, W.; Baik, J.-H.; Sung, Y.-E.; Lee, J.-C. Cross-linked sulfonated poly (arylene ether sulfone) containing a flexible and hydrophobic bishydroxy perfluoropolyether cross-linker for high-performance proton exchange membrane. ACS Appl. Mater. Interfaces 2018, 10, 21788–21793. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, S.-K.; Park, J.O.; Choi, S.-W.; Kim, K.-H.; Ko, T.; Pak, C.; Lee, J.-C. Highly reinforced pore-filling membranes based on sulfonated poly (arylene ether sulfone) s for high-temperature/low-humidity polymer electrolyte membrane fuel cells. J. Membr. Sci. 2017, 537, 11–21. [Google Scholar] [CrossRef]
- Kim, K.; Choi, S.-W.; Park, J.O.; Kim, S.-K.; Lim, M.-Y.; Kim, K.-H.; Ko, T.; Lee, J.-C. Proton conductive cross-linked benzoxazine-benzimidazole copolymers as novel porous substrates for reinforced pore-filling membranes in fuel cells operating at high temperatures. J. Membr. Sci. 2017, 536, 76–85. [Google Scholar]
- Han, J.; Kim, K.; Kim, J.; Kim, S.; Choi, S.-W.; Lee, H.; Kim, J.-J.; Kim, T.-H.; Sung, Y.-E.; Lee, J.-C. Cross-linked highly sulfonated poly (arylene ether sulfone) membranes prepared by in-situ casting and thiol-ene click reaction for fuel cell application. J. Membr. Sci. 2019, 579, 70–78. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Chakrabarty, T.; Shahi, V.K. Highly charged and stable cross-linked 4, 4′-bis (4-aminophenoxy) biphenyl-3, 3′-disulfonic acid (BAPBDS)-sulfonated poly (ether sulfone) polymer electrolyte membranes impervious to methanol. J. Mater. Chem. 2010, 20, 8036–8044. [Google Scholar] [CrossRef]
- Mukherjee, R.; Mandal, A.K.; Banerjee, S. Sulfonated poly (arylene ether sulfone) functionalized polysilsesquioxane hybrid membranes with enhanced proton conductivity. e-Polymers 2020, 20, 430–442. [Google Scholar] [CrossRef]
- Chen, R.; Jin, J.; Yang, S.; Li, G. Effect of pendant group containing fluorine on the properties of sulfonated poly (arylene ether sulfone) s as proton exchange membrane. J. Mater. Sci. 2017, 52, 1028–1038. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Han, J.; Lee, H.; Kim, H.; Kim, S.; Sung, Y.E.; Lee, J.-C. End-group cross-linked membranes based on highly sulfonated poly (arylene ether sulfone) with vinyl functionalized graphene oxide as a cross-linker and a filler for proton exchange membrane fuel cell application. J. Polym. Sci. 2020, 58, 3456–3466. [Google Scholar] [CrossRef]
- Han, J.; Zhu, L.; Pan, J.; Zimudzi, T.J.; Wang, Y.; Peng, Y.; Hickner, M.A.; Zhuang, L. Elastic long-chain multication cross-linked anion exchange membranes. Macromolecules 2017, 50, 3323–3332. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.; Zhang, L.; Li, M.; Ni, J.; Wang, S.; Ma, W.; Liu, Z.; Na, H. Cross-linked proton exchange membranes for direct methanol fuel cells: Effects of the cross-linker structure on the performances. Int. J. Hydrogen Energy 2012, 37, 12586–12596. [Google Scholar] [CrossRef]
- Rao, H.; Zhang, Z.; Liu, F. Enhanced mechanical properties and blood compatibility of PDMS/liquid crystal cross-linked membrane materials. J. Mech. Behav. Biomed. Mater. 2013, 20, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.-Y.; Kweon, O.-H.; Kim, K.-E.; Hwang, G.-J.; Moon, S.-B.; Kang, A.-S. Covalently cross-linked sulfonated poly (ether ether ketone)/tungstophosphoric acid composite membranes for water electrolysis application. J. Power Sources 2008, 181, 127–134. [Google Scholar] [CrossRef]
- Saito, T.; Nohara, T.; Tabata, K.; Nakazaki, H.; Makino, T.; Matsuo, Y.; Sato, K.; Abadie, C.; Masuhara, A. Relationship between the Proton Conductive Performance and Water Uptake Ratio on a Filler-Filled Polymer Electrolyte Membrane. Energy Fuels 2022, 36, 13924–13929. [Google Scholar] [CrossRef]
- Ueda, M.; Toyota, H.; Ouchi, T.; Sugiyama, J.I.; Yonetake, K.; Masuko, T.; Teramoto, T. Synthesis and characterization of aromatic poly (ether sulfone) s containing pendant sodium sulfonate groups. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 853–858. [Google Scholar] [CrossRef]







| Polymer | Structure | Degree of Sulfonation (%) | ηinchc (dL/g) | |
|---|---|---|---|---|
| Monomer a | 1H-NMR b | |||
| SPAES |  | 70 | 66 | 1.91 |
| SPAES-Cl |  | 70 | 66 | 1.95 |
| SDT |  | 100 | >95 | 0.93 |
| DT |  | 0 | 0 | 1.06 |
| Polymer | Solvents | ||||||
|---|---|---|---|---|---|---|---|
| DMAc | NMP | DMSO | Methanol | Ethanol | THF | Acetone | |
| SPAES | S a | S | S | Sw | Sw | Sw | Sw |
| SDT-CSPAES | Sw b | Sw | Sw | Sw | Sw | I | I |
| DT-CSPAES | Sw | Sw | Sw | I c | I | I | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Hwang, S.; Jeong, Y.-G.; Choi, Y.-S.; Kim, K. Cross-Linked Sulfonated Poly(arylene ether sulfone) Membrane Using Polymeric Cross-Linkers for Polymer Electrolyte Membrane Fuel Cell Applications. Membranes 2023, 13, 7. https://doi.org/10.3390/membranes13010007
Kim J, Hwang S, Jeong Y-G, Choi Y-S, Kim K. Cross-Linked Sulfonated Poly(arylene ether sulfone) Membrane Using Polymeric Cross-Linkers for Polymer Electrolyte Membrane Fuel Cell Applications. Membranes. 2023; 13(1):7. https://doi.org/10.3390/membranes13010007
Chicago/Turabian StyleKim, Junghwan, Seansoo Hwang, Yu-Gyeong Jeong, Yong-Seok Choi, and Kihyun Kim. 2023. "Cross-Linked Sulfonated Poly(arylene ether sulfone) Membrane Using Polymeric Cross-Linkers for Polymer Electrolyte Membrane Fuel Cell Applications" Membranes 13, no. 1: 7. https://doi.org/10.3390/membranes13010007