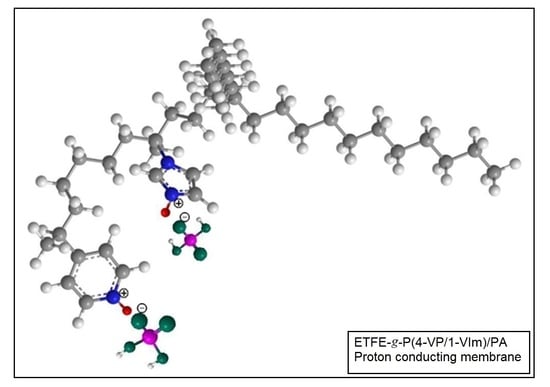
Composite Proton-Conducting Membrane with Enhanced Phosphoric Acid Doping of Basic Films Radiochemically Grafted with Binary Vinyl Heterocyclic Monomer Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Fuel Cell Test
3. Results and Discussion
3.1. Effect of Grafting Parameters on Degree of Grafting
3.2. Reactivity of 4-VP and 1-VIm in Monomer Mixture
3.3. Effect of Reactivity on Degree of Alteration in Formed Graft Copolymer
3.4. Enhancement of Acid Doping Level
3.5. Changes in Properties
3.5.1. Chemical Composition Changes
3.5.2. Morphological Changes
3.5.3. Structural Changes
3.5.4. Thermal Stability Changes
3.5.5. Mechanical Properties Changes
3.5.6. Proton Conductivity Changes with Temperature
3.5.7. Changes in Activation Energy
3.5.8. Chemical Stability Changes
3.5.9. Summary of Membrane Properties
3.6. Performance in HT-PEMFC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-Ghoul, Y.; Alminderej, F.M.; Alsubaie, F.M.; Alrasheed, R.; Almousa, N.H. Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review. Polymers 2021, 13, 4327. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A. Recent Developments of Proton Exchange Membranes for PEMFC: A Review. Front. Energy Res. 2022, 10, 1252. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Li, Y.C.; Liu, J.; He, J.; Wang, L.Y.; Lei, J. Du Recent Developments in High-Performance Nafion Membranes for Hydrogen Fuel Cells Applications. Pet. Sci. 2022, 19, 1371–1381. [Google Scholar] [CrossRef]
- Sun, X.; Simonsen, S.C.; Norby, T.; Chatzitakis, A. Composite Membranes for High Temperature PEM Fuel Cells and Electrolysers: A Critical Review. Membranes 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Zholobko, O.; Wu, X.F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects. Energies 2020, 14, 135. [Google Scholar] [CrossRef]
- Sun, C.; Zlotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Di Noto, V. [Nafion/(WO3)x] Hybrid Membranes for Vanadium Redox Flow Batteries. Solid State Ion. 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A Comprehensive Review of PBI-Based High Temperature PEM Fuel Cells. Int. J. Hydrog. Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Sulaiman, R.R.R.; Walvekar, R.; Wong, W.Y.; Khalid, M.; Pang, M.M. Proton Conductivity Enhancement at High Temperature on Polybenzimidazole Membrane Electrolyte with Acid-Functionalized Graphene Oxide Fillers. Membranes 2022, 12, 344. [Google Scholar] [CrossRef]
- Quartarone, E.; Angioni, S.; Mustarelli, P. Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review. Materials 2017, 10, 687. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Jensen, J.O.; Savinell, R.F.; Bjerrum, N.J. High Temperature Proton Exchange Membranes Based on Polybenzimidazoles for Fuel Cells. Prog. Polym. Sci. 2009, 34, 449–477. [Google Scholar] [CrossRef]
- Giffin, G.A.; Galbiati, S.; Walter, M.; Aniol, K.; Ellwein, C.; Kerres, J.; Zeis, R. Interplay between Structure and Properties in Acid-Base Blend PBI-Based Membranes for HT-PEM Fuel Cells. J. Memb. Sci. 2017, 535, 122–131. [Google Scholar] [CrossRef]
- Subianto, S. Recent Advances in Polybenzimidazole/Phosphoric Acid Membranes for High-Temperature Fuel Cells. Polym. Int. 2014, 63, 1134–1144. [Google Scholar] [CrossRef]
- MacArie, L.; Ilia, G. Poly(Vinylphosphonic Acid) and Its Derivatives. Prog. Polym. Sci. 2010, 35, 1078–1092. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.; Wang, D.; Li, Z.; Peng, X.; Liu, C.; Zheng, P. Metal Organic Frameworks Modified Proton Exchange Membranes for Fuel Cells. Front. Chem. 2020, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Çelik, S.Ü.; Bozkurt, A.; Hosseini, S.S. Alternatives toward Proton Conductive Anhydrous Membranes for Fuel Cells: Heterocyclic Protogenic Solvents Comprising Polymer Electrolytes. Prog. Polym. Sci. 2012, 37, 1265–1291. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.T.; Lee, J.H. Polymer Membranes for High Temperature Proton Exchange Membrane Fuel Cell: Recent Advances and Challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Myles, T.; Bonville, L.; Maric, R. Catalyst, Membrane, Free Electrolyte Challenges, and Pathways to Resolutions in High Temperature Polymer Electrolyte Membrane Fuel Cells. Catalysts 2017, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Zeis, R. Materials and Characterization Techniques for High-Temperature Polymer Electrolyte Membrane Fuel Cells. Beilstein J. Nanotechnol. 2015, 6, 68–83. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.I.; Yoon, K.H.; Song, M.; Peng, H.; Page, K.A.; Soles, C.L.; Yoon, D.Y. Structure and Properties of Polymer Electrolyte Membranes Containing Phosphonic Acids for Anhydrous Fuel Cells. Chem. Mater. 2012, 24, 115–122. [Google Scholar] [CrossRef]
- Asensio, J.A.; Sánchez, E.M.; Romero, P.G. Proton-Conducting Membranes Based on Benzimidazole Polymers for High-Temperature PEM Fuel Cells. A Chemical Quest. Chem. Soc. Rev. 2010, 39, 3210–3239. [Google Scholar] [CrossRef]
- Mader, J.; Xiao, L.; Schmidt, T.J.; Benicewicz, B.C. Polybenzimidazole/Acid Complexes as High-Temperature Membranes. Adv. Polym. Sci. 2008, 216, 63–124. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, Y.S.; Koo, K.K.; Kim, S.H.; Choi, S.H. Preparation of a Proton-Exchange Membrane with -SO3H Group Based on Polyethylene and Poly(Vinylidene Fluoride) Film by Radiation-Induced Graft Polymerization for Proton-Exchange Fuel Cell. J. Nanosci. Nanotechnol. 2015, 15, 6942–6948. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Drache, M.; Gohs, U.; Kunz, U.; Beuermann, S. Preparation of Polymer Electrolyte Membranes via Radiation-Induced Graft Copolymerization on Poly(Ethylene-Alt-Tetrafluoroethylene) (ETFE) Using the Crosslinker N,N′-Methylenebis(Acrylamide). Membranes 2018, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biancolli, A.L.G.; Barbosa, A.S.; Kodama, Y.; de Sousa, R.R.; Lanfredi, A.J.C.; Fonseca, F.C.; Rey, J.F.Q.; Santiago, E.I. Unveiling the Influence of Radiation-Induced Grafting Methods on the Properties of Polyethylene-Based Anion-Exchange Membranes for Alkaline Fuel Cells. J. Power Sources 2021, 512, 230484. [Google Scholar] [CrossRef]
- Hamada, T.; Zhao, Y.; Yoshimura, K.; Radulescu, A.; Ohwada, K.; Maekawa, Y. Hydrophobic Effect on Alkaline Stability of Graft Chains in Ammonium-Type Anion Exchange Membranes Prepared by Radiation-Induced Graft Polymerization. ChemistrySelect 2021, 6, 8879–8888. [Google Scholar] [CrossRef]
- Lim, K.L.; Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Selambakkannu, S.; Othman, N.A.F.; Yang, H. Radiation-Grafted Anion-Exchange Membrane for Fuel Cell and Electrolyzer Applications: A Mini Review. Membranes 2021, 11, 397. [Google Scholar] [CrossRef]
- Nasef, M.M. Radiation Grafted Ion Conducting Membranes for Electrochemical Energy Systems: Status of Developmental and Upscaled Membranes. J. Appl. Membr. Sci. Technol. 2022, 26, 51–76. [Google Scholar] [CrossRef]
- Gubler, L. Polymer Design Strategies for Radiation-Grafted Fuel Cell Membranes. Adv. Energy Mater. 2014, 4, 1300827. [Google Scholar] [CrossRef]
- Nasef, M.M.; Ali, A.A.; Saidi, H. Composite Proton Conducting Membrane by Radiation-Induced Grafting of 1-Vinylimidazole onto Poly(Ethylene-Co-Tetrafluoroethylene) and Phosphoric Acid Doping. High Perform. Polym. 2012, 25, 198–204. [Google Scholar] [CrossRef]
- Nasef, M.M.; Shamsaei, E.; Saidi, H.; Ahmad, A.; Dahlan, K.Z.M. Preparation and Characterization of Phosphoric Acid Composite Membrane by Radiation Induced Grafting of 4-Vinylpyridine onto Poly(Ethylene-Co-Tetrafluoroethylene) Followed by Phosphoric Acid Doping. J. Appl. Polym. Sci. 2013, 128, 549–557. [Google Scholar] [CrossRef]
- Shamsaei, E.; Nasef, M.M.; Saidi, H.; Yahaya, A.H. Parametric Investigations on Proton Conducting Membrane by Radiation Induced Grafting of 4-Vinylpyridine onto Poly(Vinylidene Fluoride) and Phosphoric Acid Doping. Radiochim. Acta 2014, 102, 351–362. [Google Scholar] [CrossRef]
- He, R.; Che, Q.; Sun, B. The Acid Doping Behavior of Polybenzimidazole Membranes in Phosphoric Acid for Proton Exchange Membrane Fuel Cells. Fibers Polym. 2008, 9, 679–684. [Google Scholar] [CrossRef]
- Brunetti, A.; Fontananova, E.; Donnadio, A.; Casciola, M.; Di Vona, M.L.; Sgreccia, E.; Drioli, E.; Barbieri, G. New Approach for the Evaluation of Membranes Transport Properties for Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2012, 205, 222–230. [Google Scholar] [CrossRef]
- Sithambaranathan, P.; Nasef, M.M.; Ahmad, A.; Ripin, A. Crosslinked Composite Membrane by Radiation Grafting of 4-Vinylpyridine/Triallyl-Cyanurate Mixtures onto Poly(Ethylene-Co-Tetrafluoroethylene) and Phosphoric Acid Doping. Int. J. Hydrog. Energy 2017, 42, 9333–9341. [Google Scholar] [CrossRef]
- Rajabalizadeh Mojarrad, N.; Sadeghi, S.; Yarar Kaplan, B.; Güler, E.; Alkan Gürsel, S. Metal-Salt Enhanced Grafting of Vinylpyridine and Vinylimidazole Monomer Combinations in Radiation Grafted Membranes for High-Temperature PEM Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Han, K.; Yu, J.; Zhu, H.; Wang, Z. Non-Planar Backbone Structure Polybenzimidazole Membranes with Excellent Solubility, High Proton Conductivity, and Better Anti-Oxidative for HT-PEMFCs. RSC Adv. 2016, 6, 91068–91076. [Google Scholar] [CrossRef]
- Mahmoud Nasef, M.; Shamsaei, E.; Ghassemi, P.; Ahmed Aly, A.; Hamid Yahaya, A. Optimization Strategies for Radiation Induced Grafting of 4-Vinylpyridine onto Poly(Ethylene-Co-Tetraflouroethene) Film Using Box–Behnken Design. Radiat. Phys. Chem. 2012, 81, 437–444. [Google Scholar] [CrossRef]
- Bakhshi, H.; Zohuriaan-Mehr, M.J.; Bouhendi, H.; Kabiri, K. Spectral and Chemical Determination of Copolymer Composition of Poly (Butyl Acrylate-Co-Glycidyl Methacrylate) from Emulsion Polymerization. Polym. Test. 2009, 28, 730–736. [Google Scholar] [CrossRef]
- Zakeri, M.; Abouzari-Lotf, E.; Nasef, M.M.; Ahmad, A.; Miyake, M.; Ting, T.M.; Sithambaranathan, P. Fabrication and Characterization of Supported Dual Acidic Ionic Liquids for Polymer Electrolyte Membrane Fuel Cell Applications. Arab. J. Chem. 2019, 12, 1011–1023. [Google Scholar] [CrossRef]
- Lin, K.; Wang, C.; Qiu, Z.; Yan, Y. Enhancement of Proton Conductivity Performance in High Temperature Polymer Electrolyte Membrane, Processed the Adding of Pyridobismidazole. Polymers 2022, 14, 1283. [Google Scholar] [CrossRef]
- Prasad, T.L.; Tewari, P.K.; Sathiyamoorthy, D. Parametric Studies on Radiation Grafting of Polymeric Sorbents for Recovery of Heavy Metals from Seawater. Ind. Eng. Chem. Res. 2010, 49, 6559–6565. [Google Scholar] [CrossRef]
- Hassan, M.I.U.; Taimur, S.; Khan, I.A.; Yasin, T.; Ali, S.W. Surface Modification of Polypropylene Waste by the Radiation Grafting of Styrene and Upcycling into a Cation-Exchange Resin. J. Appl. Polym. Sci. 2019, 136, 47145. [Google Scholar] [CrossRef]
- Wang, L.; Brink, J.J.; Liu, Y.; Herring, A.M.; Ponce-González, J.; Whelligan, D.K.; Varcoe, J.R. Non-Fluorinated Pre-Irradiation-Grafted (Peroxidated) LDPE-Based Anion-Exchange Membranes with High Performance and Stability. Energy Environ. Sci. 2017, 10, 2154–2167. [Google Scholar] [CrossRef] [Green Version]
- Gautam, D.; Gupta, B.; Ikram, S. Radiation-Induced Graft Copolymerization of α-Methyl Styrene and Butyl Acrylate Mixture into Polyetheretherketone Films. J. Appl. Polym. Sci. 2013, 128, 1854–1860. [Google Scholar] [CrossRef]
- Gubler, L.; Slaski, M.; Wallasch, F.; Wokaun, A.; Scherer, G.G. Radiation Grafted Fuel Cell Membranes Based on Co-Grafting of α-Methylstyrene and Methacrylonitrile into a Fluoropolymer Base Film. J. Memb. Sci. 2009, 339, 68–77. [Google Scholar] [CrossRef]
- Khedr, R.F. Synthesis of Amidoxime Adsorbent by Radiation-Induced Grafting of Acrylonitrile/Acrylic Acid on Polyethylene Film and Its Application in Pb Removal. Polymers 2022, 14, 3136. [Google Scholar] [CrossRef]
- Ali, A.A.; Nasef, M.M.; Saidi, H.; Ahmad, A. Preparation and characterization of poly(1-Vinyl imidazole)-graft-etfe/phosphoric acid proton conducting membranes. J. Teknol. 2015, 75, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Otsu, T.; Matsumoto, A.; Shiraishi, K.; Amaya, N.; Koinuma, Y. Effect of the Substituents on Radical Copolymerization of Dialkyl Fumarates with Some Vinyl Monomers. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1559–1565. [Google Scholar] [CrossRef]
- Mayo, F.R.; Lewis, F.M. Copolymerization. I. A Basis for Comparing the Behavior of Monomers in Copolymerization; The Copolymerization of Styrene and Methyl Methacrylate. J. Am. Chem. Soc. 1944, 66, 1594–1601. [Google Scholar] [CrossRef]
- Skvortsova, G.G.; Skushnikova, A.I.; Domnina, Y.S.; Brodskaya, E.I. Copolymerization of 1-vinylimidazole with 4-vinylpyridine. Polym. Sci. U.S.S.R. 1977, 19, 2398–2405. [Google Scholar] [CrossRef]
- Bisht, H.S.; Ray, S.S.; Chatterjee, A.K. Resonance, Polar, and Steric Effects of Substituent on Monomer Reactivity in Radical Polymerization of Alkyl 4-Vinylbenzoate and Butylacrylate. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 1864–1866. [Google Scholar] [CrossRef]
- Harwood, H.J. Sequence Distribution in Copolymers. Chemical Studies. Angew. Chemie Int. Ed. Engl. 1965, 4, 394–401. [Google Scholar] [CrossRef]
- Gatica, N.; Gargallo, L.; Radic, D. 2-Vinylpyridine-Co-N-Vinyl-2-Pyrrolidone and 4-Vinylpyridine-Co-N-Vinyl-2-Pyrrolidone Copolymers: Synthesis and Reactivity Ratios. Polym. Int. 1998, 45, 285–290. [Google Scholar] [CrossRef]
- Zuo, Z.; Fu, Y.; Manthiram, A. Novel Blend Membranes Based on Acid-Base Interactions for Fuel Cells. Polymers 2012, 4, 1627–1644. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, S.; Chen, H.; Li, J.; Wang, X.; Mao, T.; Wang, Z. The Impact of Poly (Ionic Liquid) on the Phosphoric Acid Stability of Polybenzimidazole-Base HT-PEMs. Renew. Energy 2021, 163, 1692–1700. [Google Scholar] [CrossRef]
- Escorihuela, J.; García-Bernabé, A.; Compañ, V. A Deep Insight into Different Acidic Additives as Doping Agents for Enhancing Proton Conductivity on Polybenzimidazole Membranes. Polymers 2020, 12, 1374. [Google Scholar] [CrossRef]















| Sample | Dehydration | PA Evaporation | Depolymerization | ETFE Degradation |
|---|---|---|---|---|
| ETFE film | - | - | - | 440 °C |
| ETFE-g-P(4-VP) | - | - | 340 °C | 470 °C |
| ETFE-g-P(4-VP/1-Vm) | 150 °C | - | 290 °C | 445 °C |
| ETFE-g-P(4-VP)/PA | 150 °C | 200 °C | 340 °C | 485 °C |
| ETFE-g-P(4-VP/1-Vm)/PA | 150 °C | 200 °C | 320 °C | 450 °C |
| Properties | P(4VP/VIm)/PA | P(4-VP)/PA | PBI/PA [29,34] |
|---|---|---|---|
| DG (%) | 59 | 59 | N/A |
| DL (%) | 119 | 97 | 61 |
| Thickness (µm) | 105 | 104 | 100 |
| Thermal stability (°C) | 200 | 200 | 200 |
| Proton conductivity at 120 °C (mS/cm) | 75.4 | 33.4 | 9.6 |
| Tensile strength (MPa) | 12.3 | 17 | 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sithambaranathan, P.; Nasef, M.M.; Ahmad, A.; Abbasi, A.; Ting, T.M. Composite Proton-Conducting Membrane with Enhanced Phosphoric Acid Doping of Basic Films Radiochemically Grafted with Binary Vinyl Heterocyclic Monomer Mixtures. Membranes 2023, 13, 105. https://doi.org/10.3390/membranes13010105
Sithambaranathan P, Nasef MM, Ahmad A, Abbasi A, Ting TM. Composite Proton-Conducting Membrane with Enhanced Phosphoric Acid Doping of Basic Films Radiochemically Grafted with Binary Vinyl Heterocyclic Monomer Mixtures. Membranes. 2023; 13(1):105. https://doi.org/10.3390/membranes13010105
Chicago/Turabian StyleSithambaranathan, Paveswari, Mohamed Mahmoud Nasef, Arshad Ahmad, Amin Abbasi, and T. M. Ting. 2023. "Composite Proton-Conducting Membrane with Enhanced Phosphoric Acid Doping of Basic Films Radiochemically Grafted with Binary Vinyl Heterocyclic Monomer Mixtures" Membranes 13, no. 1: 105. https://doi.org/10.3390/membranes13010105