Bio-Based Ceramic Membranes for Bacteria Removal from Water
Abstract
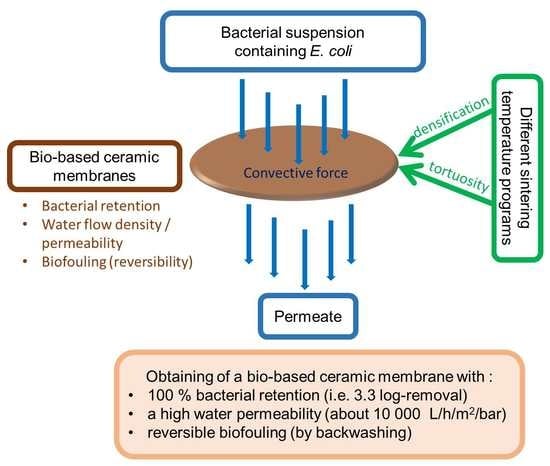
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of the Bio-Based Ceramic Membranes
2.3. Bio-Based Ceramic Membranes Characterizations
2.4. Measurements of Water and PBS Flux Densities
2.5. Bacterial Removals
2.5.1. Bacterial Suspension Preparation and Filtration
2.5.2. Bacterial Counting and Assessment of the Bacterial Removals
2.6. Biofouling and Its Mechanical Reversibility
3. Results and Discussion
3.1. Characterizations of the Bio-Based Membranes
3.2. Water and PBS Flux Densities of the Bio-Based Membranes Elaborated
3.3. Bacterial Retention Performances
3.4. Characterization of the Fouling during Filtration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN-Water. L’eau et les Changements Climatiques, Rapport Mondial des Nations Unies sur la Mise en Œuvre des Ressources en Eau; UNESCO: Paris, France, 2020; 259p. [Google Scholar]
- Ashbolt, N.J. Microbial contamination of drinking water and human health from community water systems. Curr. Environ. Health Rep. 2015, 2, 95–106. [Google Scholar] [CrossRef]
- In EPA 822-S-12-001; Edition of the Drinking Water Standards and Health Advisories. Office of Water United States Environmental Protection Agency: Washington, DC, USA, 2012; 643p.
- Burggraaf, A.J.; Cot, L. Chapter 1 General Overview, Trends and Prospects. Membr. Sci. Technol. 1996, 4, 1–20. [Google Scholar] [CrossRef]
- Saffaj, N.; Alami Younssi, S.; Albizane, A.; Messouadi, A.; Bouhria, M.; Persin, M.; Cretin, M.; Larbot, A. Preparation and characterization of ultrafiltration membranes for toxic removal from wastewater. Desalination 2004, 168, 259–263. [Google Scholar] [CrossRef]
- Mouiya, M.; Abourriche, A.; Bouazizi, A.; Benhammou, A.; El Hafiane, Y.; Abouliatim, Y.; Nibou, L. Flat ceramic microfiltration membrane based on natural clay and Moroccan Phosphate for desalination and Industrial wastewater treatment. Desalination 2018, 427, 42–50. [Google Scholar] [CrossRef]
- Kumar, C.M.; Roshni, M.; Vasanth, D. Treatment of aqueous bacterial solution using ceramic membrane prepared from cheaper clays: A detailed investigation of fouling and cleaning. J. Water Process Eng. 2019, 29, 100797. [Google Scholar] [CrossRef]
- Mohamed, M.; Njoya, D.; Hajjaji, M.; Njoya, A.; Lecomte-nana, G.L.; Njopwouo, D. Effect of porogenic agent type and firing temperatures on properties of low-cost microfiltration membranes from kaolin Trans. Ind. Ceram. Soc. 2020, 79, 5456. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Mohd, H.D.O.; Ismail, A.F.; Mukhlis, A.R.; Juhana, J.; Yuji, I.; Sawao, H.; Mohd, I.H.M.D.; Mohd, Z.M.Y. Fabrication of low cost, green silica based ceramic hollow fibre membrane prepared from waste rice husk for water filtration application. Ceram. Int. 2018, 44, 10498–10509. [Google Scholar] [CrossRef]
- Jamalludin, M.R.; Zawati, H.; Mohd, H.D.O.; Hubadillah, S.K.; Muhamad, Z.Y.; Ahmad, F. IMorphology and property study of green ceramic hollow fiber membrane derived from waste sugarcane bagasse ash (WSBA). Ceram. Int. 2018, 44, 18450–18461. [Google Scholar] [CrossRef]
- Mouiya, M.; Bouazizi, A.; Abourriche, A.; El Khessaimi, Y.; Benhammou, A.E.Y.; Taha, Y. Results in materials effect of sintering temperature on the microstructure and mechanical behavior of porous ceramics made from clay and banana peel powder. Results Mater 2019, 4, 100028. [Google Scholar] [CrossRef]
- Kamgang-syapnjeu, P.; Njoya, D.; Kamseu, E.; Cornette De Saint, L.; Marcano-zerpa, A.; Balme, S.; Bechelany, M.; Soussan, L. Elaboration of a new ceramic membrane support from Cameroonian clays, coconut husks and eggshells: Application for Escherichia Coli bacteria retention. Appl. Clay Sci. 2020, 198, 105836. [Google Scholar] [CrossRef]
- Masmoudi, S.; Larbot, A.; El Feki, H.; Ben Amar, R. Elaboration and characterisation of apatite based mineral supports for microfiltration and ultrafiltration membranes. Ceram. Int. 2007, 33, 337–344. [Google Scholar] [CrossRef]
- Majouli, A.; Alami Younssi, S.; Tahiri, S.; Albizane, A.; Loukili, H.; Belhaj, M. Characterization of flat membrane support elaborated from local Moroccan perlite. Desalination 2011, 277, 61–66. [Google Scholar] [CrossRef]
- Liyanage, C.D.; Pieris, M. A Physico-chemical analysis of coconut shell powder. Procedia Chem. 2015, 16, 222–228. [Google Scholar] [CrossRef]
- Saffaj, N.; Persin, M.; Alami Younssi, S.; Albizane, A.; Cretin, M.; Larbot, A. Elaboration and characterization of microfiltration and ultrafiltration membranes deposited on raw support prepared from natural Moroccan clay: Application to filtration of solution containing dyes and salts. Appl. Clay Sci. 2006, 31, 110–119. [Google Scholar] [CrossRef]
- El Qacimi, N.; El Baraka, N.; Saffaj, N.; Mamouni, R.; Laknifli, A.; Alami Younssi, S.; Faouzi, A.; Zidouh, H. Preparation and characterization of flat membrane support based on Sahara Moroccan lcay: Application to the Filtration of textile effluents. Desalination Water Treat. 2019, 143, 111–117. [Google Scholar] [CrossRef]
- Périnet, G. La synthèse de l’anorthite au cours de la cuisson des céramiques. Bull. Soc. Fr. Minéral. Cristallogr. 1962, 85, 120–122. [Google Scholar] [CrossRef]
- Traoré, K.; Tibo, S.K.; Philippe, B. Gehlenite and Anorthite crystallisation from Kaolinite and Calcite Mix. Ceram. Int. 2003, 29, 377–383. [Google Scholar] [CrossRef]
- Bazin, M.M.; Yuzo, N.; Norhayati, A. Chemical cleaning of microfiltration ceramic membrane fouled by nom. J. Teknol. 2018, 80, 95–103. [Google Scholar] [CrossRef]
- Matyka, M.; Koza, Z. Tortuosity-porosity relation in porous media flow. Phys. Rev. E. 2008, 78, 026306. [Google Scholar] [CrossRef]
- Sobieski, W.; Lipiński, S. The analysis of the relations between porosity and tortuosity in granular beds. Techn. Sci. 2017, 20, 75–85. [Google Scholar] [CrossRef]
- Deibert, W.; Gestel, T.V. Ceramic membranes: Materials—Components—Potential Applications. Chem. Bio. Eng. 2019, 6, 1–12. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality: Incorporating 1st and 2nd Addenda, Recommendations; World Health Organization: Geneva, Switzerland, 2008; Volume 3, 668p. [Google Scholar]
- Klosek-Wawrzyn, E.; Jan, M.; Pawel, M. Sintering behavior of kaolin with calcite. Procedia Eng. 2013, 57, 572–582. [Google Scholar] [CrossRef]
- Malaiškiene, J.; Mačiulaitis, R.M. Frost resistant ceramics produced from local raw materials and wastes. Procedia Eng. 2013, 57, 739–745. [Google Scholar] [CrossRef]
- Zhu, L.; Kadalipura, P.R.; Man, X.; Yingchao, D. Ceramic-based composite membrane with a porous network surface featuring a highly stable flux for drinking water purification. Membranes 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Kaetzl, K.; Lü, M.; Edith, N.; Stefan, K.; Marc, W. Slow sand filtration of raw wastewater using biochar as an alternative filtration media. Sci. Rep. 2020, 10, 1229. [Google Scholar] [CrossRef]
- Zhao, Y.; Guohe, H.; Chunjiang, A.; Jing, H.; Xiaying, X.; Xiujuan, C. Removal of Escherichia Coli from water using functionalized porous ceramic disk filter coated with Fe/TiO2 Nano-composites. J. Water Process Eng. 2020, 33, 101013. [Google Scholar] [CrossRef]
- Iaich, S.; Messaoudi, L. Preparation of new ceramic supports macro-porous for microfiltration and ultrafiltration membranes based Moroccan clay. IOSR J. Mech. Civ. Eng. 2014, 11, 56–62. [Google Scholar] [CrossRef]
- Saja, S.; Abdelmjid, B.; Brahim, A.; Mohamed, O.; Abderrahman, A.; Jamal, B.; Alami Younssi, S. Elaboration and characterization of low-cost ceramic membrane made from natural Moroccan perlite for treatment of industrial wastewater. J. Environ. Chem. Eng. 2018, 6, 451–458. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G. Development of ceramic membranes from Low-cost clays for the separation of oil–water emulsion. Desalination Water Treat. 2016, 57, 1927–1939. [Google Scholar] [CrossRef]




| Sintering Temperature Programs | Bio-Based Membranes Obtained |
|---|---|
| 1 °C/min up to 500 °C for 2 h and then 2 °C/min up to 900 °C for 4 h | S1 |
| 5 °C/min up to 900 °C for 2 h | S2 |
| 5 °C/min up to 1000 °C for 2 h | S3 |
| Bio-Based Membranes | Porosity (%) | Mean Pore Diameters (µm) |
|---|---|---|
| S1 | 51.9 ± 0.1 | 0.083 ± 0.001 |
| S2 | 28.2 ± 0.1 | 0.053 ± 0.002 |
| S3 | 31.2 ± 0.1 | 0.060 ± 0.001 |
| Bio-Based Membranes | LRV (log) | P (%) |
|---|---|---|
| S1 | 1.0 ± 0.1 | 90.4 ± 0.2 |
| S2 | 0.7 ± 0.0 | 80.2 ± 0.5 |
| S3 | 3.3 ± 0.3 | 99.8 ± 0.3 |
| Authors | Materials Used | Retention Performances (%) |
|---|---|---|
| [7] | Ceramic membrane (China clay, quartz and calcium carbonate) | 90.24% of E. coli |
| [27] | Ceramic-based composite membrane (mullite–carbon nanotubes) | 100% of E. coli 100% of S. aureus |
| [28] | Miscanthus biochar filters Sand filters | 1.35 ± 0.27 log of E. coli 1.18 ± 0.31 log of E. coli |
| [29] | Fe/TiO2 membrane | 99.99% of E. coli |
| Our work | Bio-based membrane (clays, coconut husks and eggshells) | 100% of E. coli (3.3 log) |
| Membranes | Water Flux (L/h.m2) | Water Flux after Backwashing (L/h.m2) | Water Loss Rate (PF) (%) |
|---|---|---|---|
| S1 | 2843 ± 92 | 2701 ± 32 | 5.0 ± 0.1 |
| S2 | 1062 ± 9 | 1062 ± 19 | 0 |
| S3 | 2123 ± 72 | 2123 ± 22 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamgang-Syapnjeu, P.; Njoya, D.; Kamseu, E.; Balme, S.; Bechelany, M.; Soussan, L. Bio-Based Ceramic Membranes for Bacteria Removal from Water. Membranes 2022, 12, 901. https://doi.org/10.3390/membranes12090901
Kamgang-Syapnjeu P, Njoya D, Kamseu E, Balme S, Bechelany M, Soussan L. Bio-Based Ceramic Membranes for Bacteria Removal from Water. Membranes. 2022; 12(9):901. https://doi.org/10.3390/membranes12090901
Chicago/Turabian StyleKamgang-Syapnjeu, Pelagie, Dayirou Njoya, Elie Kamseu, Sebastien Balme, Mikhael Bechelany, and Laurence Soussan. 2022. "Bio-Based Ceramic Membranes for Bacteria Removal from Water" Membranes 12, no. 9: 901. https://doi.org/10.3390/membranes12090901