pH and pCl Operational Parameters in Some Metallic Ions Separation with Composite Chitosan/Sulfonated Polyether Ether Ketone/Polypropylene Hollow Fibers Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.1.1. Reagents
2.1.2. Materials
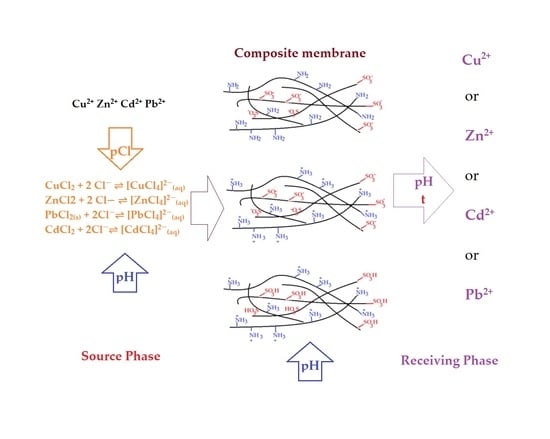
2.2. Procedures
2.2.1. Sulfonated Polyether Ether Ketone (sPEEK) Preparation
2.2.2. Obtaining Composite Membranes
2.2.3. Separation Techniques
2.3. Performances Materials, Membranes, and Processes Determination Procedure
2.4. Equipment
3. Results and Discussions
3.1. Morphological, Structural and Thermal Characterization of the Obtained Composite Membranes
3.1.1. Scanning Electron Microscopy Study
3.1.2. Fourier Transform InfraRed (FTIR) and UV-Vis Spectrometry Analysis
3.1.3. Thermal Behaviour of Membrane Materials and Composite Membrane
3.2. Preliminary Ion Separation Tests with Prepared Composite Membranes
3.2.1. Nanofiltration Separation of Binary Systems with Composite Membranes from 3 mol/L Hydrochloric Acid Solutions
3.2.2. Pertraction Separation of Binary Systems with Composite Membranes from 3 mol/L Hydrochloric Acid Solutions
3.2.3. Separation of Quaternary Systems with Composite Membranes by Nanofiltration
- The separation of zinc and cadmium ions showed the narrowest variation range, most likely because these ions were retained to the same extent either by the cationic or anionic groups of the composite membrane.
- Copper separation was excellent at pCl and pH values as high as possible.
- The separation of lead in environments with sufficiently low pCl was little-influenced by the pH value. However, caution is advised when both pH and pCl are high.
- At high pH values, the separation of copper, zinc, and cadmium ions was very good because they interacted with the membrane in both sulfonic and amino groups.
- Tracking the influence of the molecular weight of chitosan;
- Degree of sulfonation of the poly-ether-ether-ketone;
- Decreasing the thickness of the polymer membrane support layer;
- Optimization of flow-retention under the imposed pressure limit conditions.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoettecke, L.; Thiem, S.; Schäfer, J.; Niessen, S. Resilience optimization of multi-modal energy supply systems: Case study in German metal industry. Comput. Chem. Eng. 2022, 162, 107824. [Google Scholar] [CrossRef]
- Jeong, H.; Choi, J.Y.; Lee, J.; Lim, J.; Ra, K. Heavy metal pollution by road-deposited sediments and its contribution to total suspended solids in rainfall runoff from intensive industrial areas. Environ. Pollut. 2020, 265, 115028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, X.; Wang, L. Spatial distribution and source analysis of heavy metals in soils influenced by industrial enterprise distribution: Case study in Jiangsu Province. Sci. Total Environ. 2020, 710, 134953. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Chen, X. Environmental regulation and energy-environmental performance—empirical evidence from China’s non-ferrous metals industry. J. Environ. Manag. 2020, 269, 110722. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, J.; Wang, L.; Liang, T.; Guo, Q.; Guan, Y.; Rinklebe, J. Elucidating the differentiation of soil heavy metals under different land uses with geographically weighted regression and self-organizing map. Environ. Pollut. 2020, 260, 114065. [Google Scholar] [CrossRef]
- Sodango, T.H.; Li, X.; Sha, J.; Bao, Z. Review of the spatial distribution, source and extent of heavy metal pollution of soil in China: Impacts and mitigation approaches. J. Health Pollut. 2018, 8, 53–70. [Google Scholar] [CrossRef]
- Mukherjee, I.; Singh, U.K.; Singh, R.P. An overview on heavy metal contamination of water system and sustainable approach for remediation. Water Pollut. Manag. Pract. 2021, 255–277. [Google Scholar] [CrossRef]
- Long, Z.; Huang, Y.; Zhang, W.; Shi, Z.; Yu, D.; Chen, Y.; Liu, C.; Wang, R. Effect of different industrial activities on soil heavy metal pollution, ecological risk, and health risk. Environ. Monit. Assess. 2021, 193, 20. [Google Scholar] [CrossRef]
- Shezi, B.; Street, R.A.; Webster, C.; Kunene, Z.; Mathee, A. Heavy Metal Contamination of Soil in Preschool Facilities around Industrial Operations, Kuils River, Cape Town (South Africa). Int. J. Environ. Res. Public Health 2022, 19, 4380. [Google Scholar] [CrossRef]
- Zakaria, Z.; Zulkafflee, N.S.; Mohd Redzuan, N.A.; Selamat, J.; Ismail, M.R.; Praveena, S.M.; Tóth, G.; Abdull Razis, A.M.C. Understanding Potential Heavy Metal Contamination, Absorption, Translocation and Accumulation in Rice and Human Health Risks. Plants 2021, 10, 1070. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Flotation in Water and Wastewater Treatment. Processes 2018, 6, 116. [Google Scholar] [CrossRef]
- Zamboulis, D.; Peleka, E.N.; Lazaridis, N.K.; Matis, K.A. Metal ion separation and recovery from environmental sources using various flotation and sorption techniques. J. Chem. Technol. Biotechnol. 2011, 86, 335–344. [Google Scholar] [CrossRef]
- Gunarathne, V.; Rajapaksha, A.U.; Vithanage, M.; Alessi, D.S.; Selvasembian, R.; Naushad, M.; You, S.; Oleszczuk, P.; Ok, Y.S. Hydrometallurgical processes for heavy metals recovery from industrial sludges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1022–1062. [Google Scholar] [CrossRef]
- Vidu, R.; Matei, E.; Predescu, A.M.; Alhalaili, B.; Pantilimon, C.; Tarcea, C.; Predescu, C. Removal of Heavy Metals from Wastewaters: A Challenge from Current Treatment Methods to Nanotechnology Applications. Toxics 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, D.; Zhao, Y.; Zhao, R.; Russell, S.J.; Ning, X. A Review on Nanocellulose and Superhydrophobic Features for Advanced Water Treatment. Polymers 2022, 14, 2343. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; p. 541. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, S.; Albarakaty, F.M.; Kalia, A.; Hassan, M.M.; Abd-Elsalam, K.A. Biosorption and Bioleaching of Heavy Metals from Electronic Waste Varied with Microbial Genera. Sustainability 2022, 14, 935. [Google Scholar] [CrossRef]
- Fertu, D.I.; Bulgariu, L.; Gavrilescu, M. Modeling and Optimization of Heavy Metals Biosorption by Low-Cost Sorbents Using Response Surface Methodology. Processes 2022, 10, 523. [Google Scholar] [CrossRef]
- Baby, R.; Hussein, M.Z.; Abdullah, A.H.; Zainal, Z. Nanomaterials for the Treatment of Heavy Metal Contaminated Water. Polymers 2022, 14, 583. [Google Scholar] [CrossRef]
- Li, M.; Kuang, S.; Kang, Y.; Ma, H.; Dong, J.; Guo, Z. Recent advances in application of iron-manganese oxide nanomaterials for removal of heavy metals in the aquatic environment. Sci. Total Environ. 2022, 819, 153157. [Google Scholar] [CrossRef]
- Pooja, G.; Kumar, P.S.; Indraganti, S. Recent advancements in the removal/recovery of toxic metals from aquatic system using flotation techniques. Chemosphere 2022, 287, 132231. [Google Scholar] [CrossRef]
- Nallakukkala, S.; Rehman, A.U.; Zaini, D.B.; Lal, B. Gas Hydrate-Based Heavy Metal Ion Removal from Industrial Wastewater: A Review. Water 2022, 14, 1171. [Google Scholar] [CrossRef]
- Huang, H.; Xia, C.; Liang, D.; Xie, Y.; Kong, F.; Fu, J.; Dou, Z.; Yang, Q.; Suo, W.; Zhang, Q.; et al. Removal and magnetic recovery of heavy metals and pesticides from soil by layered double hydroxides modified biotite. Chem. Eng. J. 2022, 431, 134113. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Yu, D.; Liu, J.; Zhao, L.; Liu, J.; Liu, S. Preparation of Magnetic MIL-68(Ga) Metal–Organic Framework and Heavy Metal Ion Removal Application. Molecules 2022, 27, 3443. [Google Scholar] [CrossRef] [PubMed]
- Jean, E.; Villemin, D.; Hlaibi, M.; Lebrun, L. Heavy metal ions extraction using new supported liquid membranes containing ionic liquid as carrier. Sep. Purif. Technol. 2018, 201, 1–9. [Google Scholar] [CrossRef]
- Rongwong, W.; Goh, K. Resource recovery from industrial wastewaters by hydrophobic membrane contactors: A review. J. Environ. Chem. Eng. 2020, 8, 104242. [Google Scholar] [CrossRef]
- Yesil, H.; Molaey, R.; Calli, B.; Tugtas, A.E. Removal and recovery of heavy metals from sewage sludge via three-stage integrated process. Chemosphere 2021, 280, 130650. [Google Scholar] [CrossRef] [PubMed]
- Zulkefeli, N.S.W.; Weng, S.K.; Abdul Halim, N.S. Removal of heavy metals by polymer inclusion membranes. Curr. Pollut. Rep. 2018, 4, 84–92. [Google Scholar] [CrossRef]
- Alotaibi, A.A.; Shukla, A.K.; Mrad, M.H.; Alswieleh, A.M.; Alotaibi, K.M. Fabrication of Polysulfone-Surface Functionalized Mesoporous Silica Nanocomposite Membranes for Removal of Heavy Metal Ions from Wastewater. Membranes 2021, 11, 935. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.D.C.B.S.M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Imdad, S.; Dohare, R.K. A critical review on heavy metals removal using ionic liquid membranes from the industrial wastewater. Chem. Eng. Processing-Process Intensif. 2022, 173, 108812. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Kurniawan, T.A.; Puteh, M.H.; Ismail, A.M.C.; Khongnakorn, W.; Rahman, M.A.; Jaafar, J. Advances in adsorptive membrane technology for water treatment and resource recovery applications: A critical review. J. Environ. Chem. Eng. 2022, 10, 107633. [Google Scholar] [CrossRef]
- Ezziat, L.; Elabed, A.; Ibnsouda, S.; El Abed, S. Challenges of microbial fuel cell architecture on heavy metal recovery and removal from wastewater. Front. Energy Res. 2019, 7, 1. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Kavitha, E.; Poonguzhali, E.; Nanditha, D.; Kapoor, A.; Arthanareeswaran, G.; Prabhakar, S. Current status and future prospects of membrane separation processes for value recovery from wastewater. Chemosphere 2022, 291, 132690. [Google Scholar] [CrossRef]
- Liao, Z.; Zhu, J.; Li, X.; Van der Bruggen, B. Regulating composition and structure of nanofillers in thin film nanocomposite (TFN) membranes for enhanced separation performance: A critical review. Sep. Purif. Technol. 2021, 266, 118567. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.M.C.; Tan, Y.H.; Chong, C.Y.; Krause-Rehberg, R.; Awad, S. Tailor-made thin film nanocomposite membrane incorporated with graphene oxide using novel interfacial polymerization technique for enhanced water separation. Chem. Eng. J. 2018, 344, 524–534. [Google Scholar] [CrossRef]
- Abdelsamad, A.M.A.; Khalil, A.S.G.; Ulbricht, M. Influence of controlled functionalization of mesoporous silica nanoparticles as tailored fillers for thin-film nanocomposite membranes on desalination performance. J. Membr. Sci. 2018, 563, 149–161. [Google Scholar] [CrossRef]
- Demirbas, A.; Pehlivan, E.; Gode, F.; Altun, T.; Arslan, G. Adsorption of Cu(II), Zn(II), Ni(II), Pb(II), and Cd(II) from aqueous solution on amberlite IR-120 synthetic resin. J. Colloid Interface Sci. 2005, 282, 20–25. [Google Scholar] [CrossRef]
- Ali Redha, A. Removal of heavy metals from aqueous media by biosorption. Arab J. Basic Appl. Sci. 2020, 27, 183–193. [Google Scholar] [CrossRef]
- Wijers, M.C.; Jin, M.; Wessling, M.; Strathmann, H. Supported liquid membranes modification with sulphonated poly (ether ether ketone): Permeability, selectivity and stability. J. Membr. Sci. 1998, 147, 117–130. [Google Scholar] [CrossRef]
- Gohil, G.S.; Nagarale, R.K.; Binsu, V.V.; Shahi, V.K. Preparation and characterization of monovalent cation selective sulfonated poly (ether ether ketone) and poly (ether sulfone) composite membranes. J. Colloid Interface Sci. 2006, 298, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kumar, D. Chitosan-based membranes for wastewater desalination and heavy metal detoxification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 799–814. [Google Scholar] [CrossRef]
- Alharbi, H.F.; Haddad, M.Y.; Aijaz, M.O.; Assaifan, A.K.; Karim, M.R. Electrospun Bilayer PAN/Chitosan Nanofiber Membranes Incorporated with Metal Oxide Nanoparticles for Heavy Metal Ion Adsorption. Coatings 2020, 10, 285. [Google Scholar] [CrossRef]
- Croitoru, A.-M.; Ficai, A.; Ficai, D.; Trusca, R.; Dolete, G.; Andronescu, E.; Turculet, S.C. Chitosan/Graphene Oxide Nanocomposite Membranes as Adsorbents with Applications in Water Purification. Materials 2020, 13, 1687. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Karoor, S.; Sirkar, K.K. Gas absorption studies in microporous hollow fiber membrane modules. Ind. Eng. Chem. Res. 1993, 32, 674–684. [Google Scholar] [CrossRef]
- Mat, N.C.; Lou, Y.; Lipscomb, G.G. Hollow fiber membrane modules. Curr. Opin. Chem. Eng. 2014, 4, 18–24. [Google Scholar] [CrossRef]
- Wan, C.F.; Yang, T.; Lipscomb, G.G.; Stookey, D.J.; Chung, T.S. Design and fabrication of hollow fiber membrane modules. J. Membr. Sci. 2017, 538, 96–107. [Google Scholar] [CrossRef]
- Salamanca, M.; López-Serna, R.; Palacio, L.; Hernandez, A.; Prádanos, P.; Peña, M. Ecological Risk Evaluation and Removal of Emerging Pollutants in Urban Wastewater by a Hollow Fiber Forward Osmosis Membrane. Membranes 2022, 12, 293. [Google Scholar] [CrossRef]
- Sharma, A.K.K.; Conover, S.P.P.; Sirkar, K.K.K. Plasma Polymerized Coatings on Hollow Fiber Membranes-Applications and Their Aging Characteristics in Different Media. Membranes 2022, 12, 656. [Google Scholar] [CrossRef]
- Alashkar, A.; Al-Othman, A.; Tawalbeh, M.; Qasim, M. A Critical Review on the Use of Ionic Liquids in Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. pH-Induced 3D Printable Chitosan Hydrogels for Soft Actuation. Polymers 2022, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, A.C.; Ruse, E.; Nechifor, G.; Serban, B. Membrane materials. II. Electrodialysis with membranes of chemically modified polyetherketones. Rev. Chim. 2002, 53, 472–482. [Google Scholar]
- Baicea, C.; Nechifor, A.C.; Vaireanu, D.I.; Gales, O.; Trusca, R.; Voicu, S.I. Sulfonated poly (ether ether ketone)–activated polypyrrole composite membranes for fuel cells. Optoelectron. Adv. Mater.-Rapid Commun. 2011, 5, 1181–1185. [Google Scholar]
- Din, I.S.; Cimbru, A.M.; Rikabi, A.A.K.K.; Tanczos, S.K.; Ticu Cotorcea, S.; Nechifor, G. Iono-molecular Separation with Composite Membranes VI. Nitro-phenol separation through sulfonated polyether ether ketone on capillary polypropylene membranes. Rev. Chim. 2018, 69, 1603–1607. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Cotorcea, S.; Bungău, C.; Albu, P.C.; Pașcu, D.; Oprea, O.; Grosu, A.R.; Pîrțac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes 2021, 11, 256. [Google Scholar] [CrossRef]
- Xue, Y.; Fu, R.; Xu, T. Preparation of speek and speek/chitosan composite proton-exchange membranes for application in direct methanol fuel cells. Acta Polym. Sin. 2010, 3, 285–291. Available online: http://scholarbank.nus.edu.sg/handle/10635/64463 (accessed on 30 April 2022). [CrossRef]
- Palacio, L.; Pradanos, P.; Calvo, J.I.; Hernandez, A. Porosity measurements by a gas penetration method and other techniques applied to membrane characterization. Thin Solid Film. 1999, 348, 22–29. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Greenberg, A.R.; Peterson, M.L. Non-invasive measurement of membrane morphology via UFDR: Pore-size characterization. J. Membr. Sci. 2004, 239, 143–154. [Google Scholar] [CrossRef]
- Dimulescu, I.A.; Nechifor, A.C.; Bǎrdacǎ, C.; Oprea, O.; Paşcu, D.; Totu, E.E.; Albu, P.C.; Nechifor, G.; Bungău, S.G. Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes. Nanomaterials 2021, 11, 1204. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Ruse, E.; Nechifor, G. Membrane materials. I. Polyetherketones. Rev. Chim. 2001, 52, 531–540. [Google Scholar]
- Gheorghe, E.; Barbu, L.; Nechifor, G.; Luca, C. The Pb2+ cations transport through liquid membrane with macro cycle benzo-18-crown-6. Rev. Chim. 2006, 57, 940–944. [Google Scholar]
- Batrinescu, G.; Scutariu, R.E.; Nechifor, G.; Ionescu, I.A.; Iancu, V.I. Comparative analysis of the processes of collagen concentration by ultrafiltration using different types of membranes. J. Appl. Polym. Sci. 2021, 138, 50055. [Google Scholar] [CrossRef]
- Nechifor, G.; Totu, E.E.; Nechifor, A.C.; Constantin, L.; Constantin, A.M.; Cărăuşu, M.E.; Isildak, I. Added value recyclability of glass fiber waste as photo-oxidation catalyst for toxic cytostatic micropollutants. Sci. Rep. 2020, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, A.C.; Pîrțac, A.; Albu, P.C.; Grosu, A.R.; Dumitru, F.; Dimulescu (Nica), I.A.; Oprea, O.; Pașcu, D.; Nechifor, G.; Bungău, S.G. Recuperative Amino Acids Separation through Cellulose Derivative Membranes with Microporous Polypropylene Fiber Matrix. Membranes 2021, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, G.; Păncescu, F.M.; Grosu, A.R.; Albu, P.C.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Pîrțac, A.; Nechifor, A.C. Osmium Nanoparticles-Polypropylene Hollow Fiber Membranes Applied in Redox Processes. Nanomaterials 2021, 11, 2526. [Google Scholar] [CrossRef]
- Nechifor, G.; Eftimie Totu, E.; Nechifor, A.C.; Isildak, I.; Oprea, O.; Cristache, C.M. Non-Resorbable Nanocomposite Membranes for Guided Bone Regeneration Based on Polysulfone-Quartz Fiber Grafted with Nano-TiO2. Nanomaterials 2019, 9, 985. [Google Scholar] [CrossRef] [PubMed]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Bungău, C.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Păncescu, F.M.; Nechifor, G. Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes 2021, 11, 445. [Google Scholar] [CrossRef]
- Gill, N.S.; Taylor, F.B. Tetrahalo Complexes of Dipositive Metals in the First Transition Series. Inorg. Synth. 1967, 9, 136–142. [Google Scholar] [CrossRef]
- Liddell, K.C.; Bautista, R.G. Equilibrium species concentrations in the aqueous HCl-NaCl-ZnCl2 and HCl-NaCl-CdCl2 systems: The effect of ionic strength. Hydrometallurgy 1988, 21, 113–124. [Google Scholar] [CrossRef]
- Szczepański, P.; Guo, H.; Dzieszkowski, K.; Rafiński, Z.; Wolan, A.; Fatyeyeva, K.; Kujawa, J.; Kujawski, W. New reactive ionic liquids as carriers in polymer inclusion membranes for transport and separation of Cd (II), Cu (II), Pb (II), and Zn (II) ions from chloride aqueous solutions. J. Membr. Sci. 2021, 638, 119674. [Google Scholar] [CrossRef]
- Suhalim, N.S.; Kasim, N.; Mahmoudi, E.; Shamsudin, I.J.; Mohammad, A.W.; Mohamed Zuki, F.; Jamari, N.L.-A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials 2022, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Vaivars, G.; Krūkle-Bērziņa, K.; Markus, M. Modelling IR Spectra of Sulfonated Polyether Ether Ketone (SPEEK) Membranes for Fuel Cells. Key Eng. Mater. 2020, 850, 138–143. [Google Scholar] [CrossRef]
- El-Araby, R.; Attia, N.K.; Eldiwani, G.; Khafagi, M.G.; Sobhi, S.; Mostafa, T. Characterization and Sulfonation Degree of Sulfonated PolyEther Ether Ketone Using Fourier Transform Infrared Spectroscopy. World Appl. Sci. J. 2014, 32, 2239–2244. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Tsuchida, E.; Choe, Y.-K.; Ikeshoji, T.; Abdul Barique, M.; Ohira, A. Ab initio studies on the proton dissociation and infrared spectra of sulfonated poly(ether etherketone) (SPEEK) membranes. Phys. Chem. Chem. Phys. 2014, 16, 1041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Yang, Y.; Ma, T. Evaluation the Resistance Growth of Aged Vehicular Proton Exchange Membrane Fuel Cell Stack by Distribution of Relaxation Times. Sustainability 2022, 14, 5677. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Truşcă, R.-D.; Ilie, C.-I.; Oprea, O.-C.; Andronescu, E. Innovative Antimicrobial Chitosan/ZnO/Ag NPs/Citronella Essential Oil Nanocomposite—Potential Coating for Grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef]
- Martell, A.E.; Smith, R.M. Critical Stability Constants; First Supplement; Plenum Press: New York, NY, USA, 1982; Volume 5, p. 419. [Google Scholar]
- Fedorov, V.A.; Kuznechikhina, M.A.; Kanarsh, I.V.; Kirnyuk, G.M.; Chernikova, G.E. Formation of mixed zinc halide complexes in aqueous solutions. Sov. J. Coord. Chem. 1978, 4, 33–38. [Google Scholar]

















| Polymer Compounds | Symbol | Molar Mass (Da) | Solubility | pKa * |
|---|---|---|---|---|
 | PEEK | 30.000 | Sulfuric acid | - |
 | sPEEK | Organic polar solvents | 1.9 | |
 | Chi | Acidulated water | 6.5 |
| Module Type | Module Length (cm) | Module Diameter (cm) | Operating Pressure (bar) | Membrane Length (cm) | Membrane Surface (m2) | Feed Solution Flow (mL/min) |
|---|---|---|---|---|---|---|
| Hollowfiber membrane | 75 ± 1.0 | 6 ± 0.1 | 1–10 | 75 ± 1.0 | 1.0 ± 0.1 | 100–1000 |
| Metallic Ion | Ionic Radius (Å) | [MCl4]2− pK instability | Precipitation Hydroxide pH | MCl2 Solubility in Water (g/100 mL) | Ks M(OH)2 |
|---|---|---|---|---|---|
| Cu2+ | 1.96 | 5.30 | 4.4 | 75.7 | 1·10−20 |
| Zn2+ | 0.83 | 0.15 | 6.8 | 432.0 | 5·10−17 |
| Cd2+ | 0.94 | 2.46 | 4.5 | 119.6 | 1·10−14 |
| Pb2+ | 1.81 | 13.22 | 4.2 | 0.99 | 3·10−16 |
| Feed Solution Characteristics | Metallic Ion Retention R (%) | ||||
|---|---|---|---|---|---|
| pH | pCl | Cu2+ | Zn2+ | Cd2+ | Pb2+ |
| 0 | 0 | 23.12 | 75.33 | 74.67 | 87.58 |
| 1 | 0 | 29.34 | 62.42 | 70.84 | 89.23 |
| 4 | 0 | 48.90 | 45.20 | 54.08 | 92.00 |
| 6 | 0 | 79.85 | 40.48 | 59.65 | 90.23 |
| 8 | 0 | 93.32 | 64.84 | 61.20 | 70.05 |
| 1 | 1 | 18.56 | 60.32 | 65.44 | 85.00 |
| 4 | 4 | 68.26 | 49.86 | 51.62 | 60.88 |
| 6 | 6 | 90.45 | 62.95 | 74.32 | 50.08 |
| 8 | 8 | 95.18 | 78.59 | 80.51 | 48.82 |
| Pressure (bar) | Flux of Pure Water (L/m2·h) | ||
|---|---|---|---|
| PPHF | sPEEK/PPHF | Chi/sPEEK/PPHF | |
| 1.0 | 8.20 | - | - |
| 1.5 | 12.37 | - | - |
| 2.0 | 19.90 | - | - |
| 2.5 | 22.85 | - | - |
| 3.0 | - | 2.32 | - |
| 3.5 | - | 3.48 | 0.63 |
| 4.0 | - | 5.95 | 1.45 |
| 5.0 | - | 6.64 | 2.32 |
| 6.0 | - | 7.22 | 2.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimbru, A.M.; Rikabi, A.A.K.K.; Oprea, O.; Grosu, A.R.; Tanczos, S.-K.; Simonescu, M.C.; Pașcu, D.; Grosu, V.-A.; Dumitru, F.; Nechifor, G. pH and pCl Operational Parameters in Some Metallic Ions Separation with Composite Chitosan/Sulfonated Polyether Ether Ketone/Polypropylene Hollow Fibers Membranes. Membranes 2022, 12, 833. https://doi.org/10.3390/membranes12090833
Cimbru AM, Rikabi AAKK, Oprea O, Grosu AR, Tanczos S-K, Simonescu MC, Pașcu D, Grosu V-A, Dumitru F, Nechifor G. pH and pCl Operational Parameters in Some Metallic Ions Separation with Composite Chitosan/Sulfonated Polyether Ether Ketone/Polypropylene Hollow Fibers Membranes. Membranes. 2022; 12(9):833. https://doi.org/10.3390/membranes12090833
Chicago/Turabian StyleCimbru, Anca Maria, Abbas Abdul Kadhim Klaif Rikabi, Ovidiu Oprea, Alexandra Raluca Grosu, Szidonia-Katalin Tanczos, Maria Claudia Simonescu, Dumitru Pașcu, Vlad-Alexandru Grosu, Florina Dumitru, and Gheorghe Nechifor. 2022. "pH and pCl Operational Parameters in Some Metallic Ions Separation with Composite Chitosan/Sulfonated Polyether Ether Ketone/Polypropylene Hollow Fibers Membranes" Membranes 12, no. 9: 833. https://doi.org/10.3390/membranes12090833