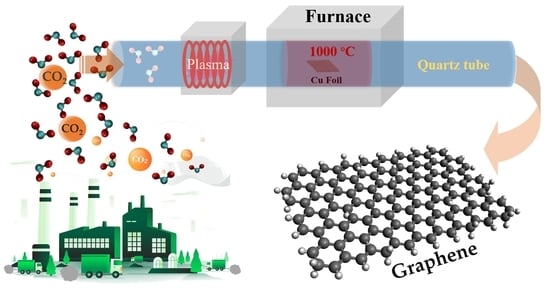
Conversion of Carbon Dioxide into Chemical Vapor Deposited Graphene with Controllable Number of Layers via Hydrogen Plasma Pre-Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Xing, Y.; Wang, Y.; Zhang, W.; Guo, Z.; Su, W. Application of iron and steel slags in mitigating greenhouse gas emissions: A review. Sci. Total Environ. 2022, 844, 157041. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Duan, X.; Moore, J.C.; Deng, X.; Luo, Y.; Huang, L.; Wang, Y. Unevenly distributed CO2 and its impacts on surface energy balance. Atmos. Res. 2022, 274, 106196. [Google Scholar] [CrossRef]
- Seesaard, T.; Goel, N.; Kumar, M.; Wongchoosuk, C. Advances in gas sensors and electronic nose technologies for agricultural cycle applications. Comput. Electron. Agric. 2022, 193, 106673. [Google Scholar] [CrossRef]
- Carnicer, J.; Alegria, A.; Giannakopoulos, C.; Di Giuseppe, F.; Karali, A.; Koutsias, N.; Lionello, P.; Parrington, M.; Vitolo, C. Global warming is shifting the relationships between fire weather and realized fire-induced CO2 emissions in Europe. Sci. Rep. 2022, 12, 10365. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Chen, J.; Abazari, R.; Adegoke, K.A.; Maxakato, N.W.; Bello, O.S.; Tahir, M.; Tasleem, S.; Sanati, S.; Kirillov, A.M.; Zhou, Y. Metal–organic frameworks and derived materials as photocatalysts for water splitting and carbon dioxide reduction. Coord. Chem. Rev. 2022, 469, 214664. [Google Scholar] [CrossRef]
- Xie, W.-H.; Li, H.; Yang, M.; He, L.-N.; Li, H.-R. CO2 capture and utilization with solid waste. Green Chem. Eng. 2022, 3, 199–209. [Google Scholar] [CrossRef]
- Arayawut, O.; Kerdcharoen, T.; Wongchoosuk, C. Structures, Electronic Properties, and Gas Permeability of 3D Pillared Silicon Carbide Nanostructures. Nanomaterials 2022, 12, 1869. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Wang, J.; Morsi, B.; Li, B. Carbon Dioxide Conversion to Nanomaterials: Methods, Applications, and Challenges. Energy Fuels 2021, 35, 11820–11834. [Google Scholar] [CrossRef]
- Kondee, S.; Arayawut, O.; Pon-On, W.; Wongchoosuk, C. Nitrogen-doped carbon oxide quantum dots for flexible humidity sensor: Experimental and SCC-DFTB study. Vacuum 2022, 195, 110648. [Google Scholar] [CrossRef]
- Chaloeipote, G.; Samarnwong, J.; Traiwatcharanon, P.; Kerdcharoen, T.; Wongchoosuk, C. High-performance resistive humidity sensor based on Ag nanoparticles decorated with graphene quantum dots. R. Soc. Open Sci. 2021, 8, 210407. [Google Scholar] [CrossRef] [PubMed]
- Traiwatcharanon, P.; Siriwatcharapiboon, W.; Jongprateep, O.; Wongchoosuk, C. Electrochemical paraquat sensor based on lead oxide nanoparticles. RSC Adv. 2022, 12, 16079–16092. [Google Scholar] [CrossRef] [PubMed]
- Kerdcharoen, T.; Wongchoosuk, C. Carbon nanotube and metal oxide hybrid materials for gas sensing. In Semiconductor Gas Sensors; Woodhead Publishing Series in Electronic and Optical Materials; Jaaniso, R., Tan, O.K., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 386–407. [Google Scholar] [CrossRef]
- Saengsonachai, A.; Seekaew, Y.; Traiwatcharanon, P.; Wongchoosuk, C. Dual functions of alternating current electroluminescent device for light emission and humidity detection. Nanotechnology 2022, 33, 405202. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Lim, W.-G.; Kang, D.; Park, J.H.; Lee, H.; Lee, J.; Lee, J.W. Transformation of carbon dioxide into carbon nanotubes for enhanced ion transport and energy storage. Nanoscale 2020, 12, 7822–7833. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, F.F.; Lau, J.; Urbina, L.G.; Licht, S. One-pot synthesis of carbon nanofibers from CO2. Nano Lett. 2015, 15, 6142–6148. [Google Scholar] [CrossRef]
- Molina-Jirón, C.; Chellali, M.R.; Kumar, C.N.S.; Kübel, C.; Velasco, L.; Hahn, H.; Moreno-Pineda, E.; Ruben, M. Direct conversion of CO2 to multi-layer graphene using Cu-Pd alloys. ChemSusChem 2019, 12, 3509–3514. [Google Scholar] [CrossRef]
- Licht, S.; Douglas, A.; Ren, J.; Carter, R.; Lefler, M.; Pint, C.L. Carbon Nanotubes Produced from Ambient Carbon Dioxide for Environmentally Sustainable Lithium-Ion and Sodium-Ion Battery Anodes. ACS Cent. Sci. 2016, 2, 162–168. [Google Scholar] [CrossRef]
- Wang, X.; Licht, G.; Liu, X.; Licht, S. One pot facile transformation of CO2 to an unusual 3-D nano-scaffold morphology of carbon. Sci. Rep. 2020, 10, 21518. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Seekaew, Y.; Arayawut, O.; Timsorn, K.; Wongchoosuk, C. Chapter Nine—Synthesis, characterization, and applications of graphene and derivatives. In Carbon-Based Nanofillers and Their Rubber Nanocomposites; Yaragalla, S., Mishra, R., Thomas, S., Kalarikkal, N., Maria, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–283. [Google Scholar] [CrossRef]
- Mazlan, N.A.; Butt, F.S.; Lewis, A.; Yang, Y.; Yang, S.; Huang, Y. The Growth of Metal–Organic Frameworks in the Presence of Graphene Oxide: A Mini Review. Membranes 2022, 12, 501. [Google Scholar] [CrossRef]
- Ghuge, A.D.; Shirode, A.R.; Kadam, V.J. Graphene: A Comprehensive Review. Curr. Drug Targets 2017, 18, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Seesaard, T.; Wongchoosuk, C. Recent Progress in Electronic Noses for Fermented Foods and Beverages Applications. Fermentation 2022, 8, 302. [Google Scholar] [CrossRef]
- Zare, P.; Aleemardani, M.; Seifalian, A.; Bagher, Z.; Seifalian, A. Graphene Oxide: Opportunities and Challenges in Biomedicine. Nanomaterials 2021, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Permatasari, F.A.; Irham, M.A.; Bisri, S.Z.; Iskandar, F. Carbon-Based Quantum Dots for Supercapacitors: Recent Advances and Future Challenges. Nanomaterials 2021, 11, 91. [Google Scholar] [CrossRef]
- Goldoni, R.; Farronato, M.; Connelly, S.T.; Tartaglia, G.M.; Yeo, W.-H. Recent advances in graphene-based nanobiosensors for salivary biomarker detection. Biosens. Bioelectron. 2021, 171, 112723. [Google Scholar] [CrossRef]
- Traiwatcharanon, P.; Siriwatcharapiboon, W.; Wongchoosuk, C. Electrochemical Sodium Ion Sensor Based on Silver Nanoparticles/Graphene Oxide Nanocomposite for Food Application. Chemosensors 2020, 8, 58. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Arunragsa, S.; Seekaew, Y.; Pon-On, W.; Wongchoosuk, C. Hydroxyl edge-functionalized graphene quantum dots for gas-sensing applications. Diam. Relat. Mater. 2020, 105, 107790. [Google Scholar] [CrossRef]
- Banerjee, A.N. Green syntheses of graphene and its applications in internet of things (IoT)—A status review. Nanotechnology 2022, 33, 322003. [Google Scholar] [CrossRef]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Botlhoko, O.J.; Setshedi, K.; Scriba, M.; Masukume, M.; Ray, S.S. Top-down synthesis of graphene: A comprehensive review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Seekaew, Y.; Phokharatkul, D.; Wisitsoraat, A.; Wongchoosuk, C. Highly sensitive and selective room-temperature NO2 gas sensor based on bilayer transferred chemical vapor deposited graphene. Appl. Surf. Sci. 2017, 404, 357–363. [Google Scholar] [CrossRef]
- Shen, C.; Yan, X.; Qing, F.; Niu, X.; Stehle, R.; Mao, S.S.; Zhang, W.; Li, X. Criteria for the growth of large-area adlayer-free monolayer graphene films by chemical vapor deposition. J. Mater. 2019, 5, 463–470. [Google Scholar] [CrossRef]
- Trinsoutrot, P.; Vergnes, H.; Caussat, B. Three dimensional graphene synthesis on nickel foam by chemical vapor deposition from ethylene. Mater. Sci. Eng. B 2014, 179, 12–16. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.; Kwon, K.W.; Park, M.-S.; Hwang, J.-H.; Kim, K.J. 3D graphene-Ni foamas an advanced electrode for high-performance nonaqueous redox flow batteries. ACS Appl. Mater. Interfaces 2017, 9, 22502–22508. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Strudwick, A.J.; Weber, N.E.; Schwab, M.G.; Kettner, M.; Weitz, R.T.; Wünsch, J.R.; Müllen, K.; Sachdev, H. Chemical Vapor Deposition of High Quality Graphene Films from Carbon Dioxide Atmospheres. ACS Nano 2015, 9, 31–42. [Google Scholar] [CrossRef]
- Xiao, T.; Hu, X.; Heng, B.; Chen, X.; Huang, W.; Tao, W.; Wang, H.; Tang, Y.; Tan, X.; Huang, X. Ni(OH)2 nanosheets grown on graphene-coated nickel foam for high-performance pseudocapacitors. J. Alloys Compd. 2013, 549, 147–151. [Google Scholar] [CrossRef]
- Jiang, F.; Fang, Y.; Xue, Q.; Chen, L.; Lu, Y. Graphene-based carbon nano-fibers grown on thin-sheet sinter-locked Ni-fiber as self-supported electrodes for supercapacitors. Mater. Lett. 2010, 64, 199–202. [Google Scholar] [CrossRef]
- Zheng, S.; Zhong, G.; Wu, X.; D’Arsiè, L.; Robertson, J. Metal-catalyst-free growth of graphene on insulating substrates by ammonia-assisted microwave plasma-enhanced chemical vapor deposition. RSC Adv. 2017, 7, 33185–33193. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Zhang, L.; Fang, Y. The SERS study of graphene deposited by gold nanoparticles with 785 nm excitation. Chem. Phys. Lett. 2013, 556, 146–150. [Google Scholar] [CrossRef]
- Klar, P.; Lidorikis, E.; Eckmann, A.; Verzhbitskiy, I.; Ferrari, A.C.; Casiraghi, C. Raman scattering efficiency of graphene. Phys. Rev. B 2013, 87, 205435. [Google Scholar] [CrossRef]
- Tu, Z.; Liu, Z.; Li, Y.; Yang, F.; Zhang, L.; Zhao, Z.; Xu, C.; Wu, S.I.; Liu, H.; Yang, H.; et al. Controllable growth of 1–7 layers of graphene by chemical vapor deposition. Caribon 2014, 73, 252–258. [Google Scholar] [CrossRef]
- Ni, Z.H.; Wang, H.M.; Kasim, J.; Fan, H.M.; Yu, T.; Wu, Y.H.; Feng, Y.P.; Shen, Z.X. Graphene Thickness Determination Using Reflection and Contrast Spectroscopy. Nano Lett. 2007, 7, 2758–2763. [Google Scholar] [CrossRef]
- Fang, L.; Yuan, W.; Wang, B.; Xiong, Y. Growth of graphene on Cu foils by microwave plasma chemical vapor deposition: The effect of in-situ hydrogen plasma post-treatment. Appl. Surf. Sci. 2016, 383, 28–32. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, B.; Wei, Z.; Luo, Z.; Wei, D.; Xi, Y.; Zhang, Y.; Liu, Y. Roles of H2 in annealing and growth times of graphene CVD synthesis over copper foil. J. Mater. Chem. A 2014, 2, 16208–16216. [Google Scholar] [CrossRef]
- Regmi, M.; Chisholm, M.F.; Eres, G. The effect of growth parameters on the intrinsic properties of large-area single layer graphene grown by chemical vapor deposition on Cu. Carbon 2012, 50, 134–141. [Google Scholar] [CrossRef]
- Son, I.H.; Song, H.J.; Kwon, S.; Bachmatiuk, A.; Lee, S.J.; Benayad, A.; Park, J.H.; Choi, J.-Y.; Chang, H.; Rümmeli, M.H. CO2 enhanced chemical vapor deposition growth of few-layer graphene over NiOx. ACS Nano 2014, 8, 9224–9232. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, Z.; Luo, Q.; Wu, Z.; Liu, L.; Shen, B.; Hu, W. Microstructure and nanoindentation behavior of Cu composites reinforced with graphene nanoplatelets by electroless co-deposition technique. Sci. Rep. 2017, 7, 1338. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Q.; Qin, Z.; Liu, L.; Wu, Z.; Shen, B.; Hu, W. Self-Assembly of Graphene-Encapsulated Cu Composites for Nonenzymatic Glucose Sensing. ACS Omega 2018, 3, 3420–3428. [Google Scholar] [CrossRef] [PubMed]
- Jang, L.-W.; Zhang, L.; Menghini, M.; Cho, H.; Hwang, J.Y.; Son, D.I.; Locquet, J.-P.; Seo, J.W. Multilayered graphene grafted copper wires. Carbon 2018, 139, 666–671. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y.; Wu, W.; Chen, W.; Gao, L.; Sun, J. A facile method to observe graphene growth on copper foil. Nanotechnology 2012, 23, 475705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Wang, B.; Zhang, H.R.; Chen, Z.Y.; Zhng, Y.; Sui, Y.P.; Li, X.L.; Xie, X.M.; Yu, G.H.; Jin, Z.; et al. The distribution of wrinkles and their effects on the oxidation resistance of chemical vapor deposition graphene. Carbon 2014, 70, 81–86. [Google Scholar] [CrossRef]
- Seekaew, Y.; Wongchoosuk, C. A novel graphene-based electroluminescent gas sensor for carbon dioxide detection. Appl. Surf. Sci. 2019, 479, 525–531. [Google Scholar] [CrossRef]
- Nakamura, M.; Kawai, T.; Irie, M.; Yuge, R.; Iijima, S.; Bandow, S.; Yudasaka, M. Graphite-like thin sheets with even-numbered layers. Carbon 2013, 61, 644–647. [Google Scholar] [CrossRef]
- Yan, Y.; Manickam, S.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of graphene oxide and graphene quantum dots from miscanthus via ultrasound-assisted mechano-chemical cracking method. Ultrason. Sonochem. 2021, 73, 105519. [Google Scholar] [CrossRef]
- Hernandez-Robles, A.; Romeu, D.; Ponce, A. On the Mechanism Controlling the Relative Orientation of Graphene Bi-Layers. Symmetry 2022, 14, 719. [Google Scholar] [CrossRef]
- Amer, M.S.; Mohammed, M.K.; Al Mafrage, A.M. Graphene to graphite; a layer by layer experimental measurements and density function theory calculations of electric conductivity. Philos. Mag. 2020, 100, 2491–2502. [Google Scholar] [CrossRef]
- Fang, X.-Y.; Yu, X.-X.; Zheng, H.-M.; Jin, H.-B.; Wang, L.; Cao, M.-S. Temperature- and thickness-dependent electrical conductivity of few-layer graphene and graphene nanosheets. Phys. Lett. A 2015, 379, 2245–2251. [Google Scholar] [CrossRef]
- Cho, J.H.; Na, S.R.; Park, S.; Akinwande, D.; Liechti, K.M.; Cullinan, M.A. Controlling the number of layers in graphene using the growth pressure. Nanotechnology 2019, 30, 235602. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.W.; Kitt, A.; Magnuson, C.W.; Hao, Y.; Ahmed, S.; An, J.; Swan, A.K.; Goldberg, B.B.; Ruoff, R.S. Transfer of CVD-Grown Monolayer Graphene onto Arbitrary Substrates. ACS Nano 2011, 5, 6916–6924. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yu, Q.; Peng, P.; Liu, Z.; Bao, J.; Pei, S.-S. Control of thickness uniformity and grain size in graphene films for transparent conductive electrodes. Nanotechnology 2012, 23, 035603. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Tsugawa, K.; Okigawa, Y.; Ishihara, M.; Yamada, T.; Hasegawa, M. Bilayer graphene synthesis by plasma treatment of copper foils without using a carbon-containing gas. Carbon 2014, 77, 823–828. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical vapor deposition of graphene-synthesis, characterisation, and applications: A Review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Luo, B.; Liu, H.; Jiang, L.; Jiang, L.; Geng, D.; Wu, B.; Hu, W.; Liu, Y.; Yu, G. Synthesis and morphology transformation of single-crystal graphene domains based on activated carbon dioxide by chemical vapor deposition. J. Mater. Chem. C 2013, 1, 2990–2995. [Google Scholar] [CrossRef]
- Hu, L.; Song, Y.; Jiao, S.; Liu, Y.; Ge, J.; Jiao, H.; Zhu, J.; Wang, J.; Zhu, H.; Fray, D.J. Direct Conversion of Greenhouse Gas CO2 into Graphene via Molten Salts Electrolysis. ChemSusChem 2016, 9, 588–594. [Google Scholar] [CrossRef]
- Svavil’Nyi, M.Y.; Panarin, V.Y.; Shkola, A.A.; Nikolenko, A.S.; Strelchuk, V.V. Plasma Enhanced Chemical Vapor Deposition synthesis of graphene-like structures from plasma state of CO2 gas. Carbon 2020, 167, 132–139. [Google Scholar] [CrossRef]
- Lu, L.; Sun, X.; Ma, J.; Yang, D.; Wu, H.; Zhang, B.; Zhang, J.; Han, B. Highly Efficient Electroreduction of CO2 to Methanol on Palladium-Copper Bimetallic Aerogels. Angew. Chem. Int. Ed. 2018, 57, 14149–14153. [Google Scholar] [CrossRef]
- Vaiano, V.; Sannino, D.; Ciambelli, P. Steam reduction of CO2 on Pd/TiO2 catalysts: A comparison between thermal and photocatalytic reactions. Photochem. Photobiol. Sci. 2015, 14, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Braeuninger-Weimer, P.; Brennan, B.; Pollard, A.J.; Hofmann, S. Understanding and Controlling Cu-Catalyzed Graphene Nucleation: The Role of Impurities, Roughness, and Oxygen Scavenging. Chem. Mater. 2016, 28, 8905–8915. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seekaew, Y.; Tammanoon, N.; Tuantranont, A.; Lomas, T.; Wisitsoraat, A.; Wongchoosuk, C. Conversion of Carbon Dioxide into Chemical Vapor Deposited Graphene with Controllable Number of Layers via Hydrogen Plasma Pre-Treatment. Membranes 2022, 12, 796. https://doi.org/10.3390/membranes12080796
Seekaew Y, Tammanoon N, Tuantranont A, Lomas T, Wisitsoraat A, Wongchoosuk C. Conversion of Carbon Dioxide into Chemical Vapor Deposited Graphene with Controllable Number of Layers via Hydrogen Plasma Pre-Treatment. Membranes. 2022; 12(8):796. https://doi.org/10.3390/membranes12080796
Chicago/Turabian StyleSeekaew, Yotsarayuth, Nantikan Tammanoon, Adisorn Tuantranont, Tanom Lomas, Anurat Wisitsoraat, and Chatchawal Wongchoosuk. 2022. "Conversion of Carbon Dioxide into Chemical Vapor Deposited Graphene with Controllable Number of Layers via Hydrogen Plasma Pre-Treatment" Membranes 12, no. 8: 796. https://doi.org/10.3390/membranes12080796