Elastic Properties of Poly(ethylene glycol) Nanomembranes and Respective Implications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Parameters of the Bulge Test
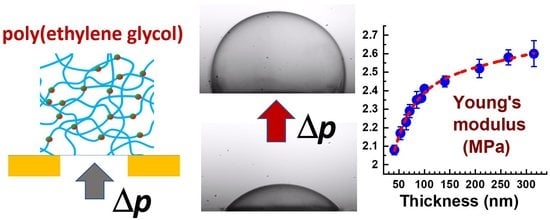
3.2. Effect of Membrane Thickness
3.3. Effect of Membrane Composition
3.4. Effect of Electron Irradiation
3.5. Effect of UV Light
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeon, S.; Lee, J.H.; Andrade, J.; De Gennes, P. Protein—Surface interactions in the presence of polyethylene oxide: I. Simplified theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Harris, J.M. (Ed.) Polyethylene Glycol Chemistry: Biotechnical and Biomedical Applications; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Prime, K.L.; Whitesides, G.M. Adsorption of proteins onto surfaces containing end-attached oligo(ethylene oxide): A model system using self-assembled monolayers. J. Am. Chem. Soc. 1993, 115, 10714–10721. [Google Scholar] [CrossRef]
- Rosenhahn, A.; Schilp, S.; Kreuzer, H.J.; Grunze, M. The role of “inert” surface chemistry in marine biofouling prevention. Phys. Chem. Chem. Phys. 2010, 12, 4275–4286. [Google Scholar] [CrossRef] [PubMed]
- Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. Factors that determine the protein resistance of oligoether self-assembled monolayers—Internal hydrophilicity, terminal hydrophilicity, and lateral packing density. J. Am. Chem. Soc. 2003, 125, 9359–9366. [Google Scholar] [CrossRef]
- Kim, S.; Gim, T.; Jeong, Y.; Ryu, J.H.; Kang, S.M. Facile Construction of Robust Multilayered PEG Films on Polydopamine-Coated Solid Substrates for Marine Antifouling Applications. ACS Appl. Mater. Interfaces 2017, 10, 7626–7631. [Google Scholar] [CrossRef]
- Cambria, E.; Renggli, K.; Ahrens, C.C.; Cook, C.; Kroll, C.; Krueger, A.T.; Imperiali, B.; Griffith, L. Covalent Modification of Synthetic Hydrogels with Bioactive Proteins via Sortase-Mediated Ligation. Biomacromolecules 2015, 16, 2316–2326. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Schuster, S.; Zharnikov, M. Chemical derivatization and biofunctionalization of hydrogel nanomembranes for potential biomedical and biosensor applications. Phys. Chem. Chem. Phys. 2016, 18, 12035–12042. [Google Scholar] [CrossRef]
- Hammer, J.; Ruta, A.; Therien, A.; West, J. Cell-compatible, site-specific covalent modification of hydrogel scaffolds enables user-defined control over cell–material interactions. Biomacromolecules 2019, 20, 2486–2493. [Google Scholar] [CrossRef]
- Larsson, A.; Ekblad, T.; Andersson, A.O.; Liedberg, B. Photografted Poly(ethylene glycol) Matrix for Affinity Interaction Studies. Biomacromolecules 2006, 8, 287–295. [Google Scholar] [CrossRef]
- Chen, R.T.; Marchesan, S.; Evans, R.; Styan, K.; Such, G.; Postma, A.; McLean, K.M.; Muir, B.; Caruso, F. Photoinitiated Alkyne–Azide Click and Radical Cross-Linking Reactions for the Patterning of PEG Hydrogels. Biomacromolecules 2012, 13, 889–895. [Google Scholar] [CrossRef]
- Cėpla, V.; Rakickas, T.; Stankevičienė, G.; Mazėtytė-Godienė, A.; Baradoke, A.; Ruželė, Ž.; Valiokas, R. Photografting and Patterning of Poly(ethylene glycol) Methacrylate Hydrogel on Glass for Biochip Applications. ACS Appl. Mater. Interfaces 2020, 12, 32233–32246. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Amirgoulova, E.V.; Ameringer, T.; Heyes, C.D.; Röcker, C.; Nienhaus, G.U.; Möller, M. Biofunctionalized, Ultrathin Coatings of Cross-Linked Star-Shaped Poly(ethylene oxide) Allow Reversible Folding of Immobilized Proteins. J. Am. Chem. Soc. 2004, 126, 4234–4239. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Ameringer, T.; Spatz, A.J.P.; Moeller, M. Ultrathin Coatings from Isocyanate-Terminated Star PEG Prepolymers: Layer Formation and Characterization. Langmuir 2005, 21, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Meyerbröker, N.; Kriesche, T.; Zharnikov, M. Novel Ultrathin Poly(ethylene glycol) Films as Flexible Platform for Biological Applications and Plasmonics. ACS Appl. Mater. Interfaces 2013, 5, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Das, S.; Zharnikov, M. Tuning the Properties of Poly(ethylene glycol) Films and Membranes by the Molecular Weight of the Precursors. ACS Appl. Polym. Mater. 2021, 4, 645–653. [Google Scholar] [CrossRef]
- Meyerbröker, N.; Zharnikov, M. Modification and Patterning of Nanometer-Thin Poly(ethylene glycol) Films by Electron Irradiation. ACS Appl. Mater. Interfaces 2013, 5, 5129–5138. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, R.; Zharnikov, M. The Effect of Ultraviolet Light on Biorepulsive Hydrogel Poly(ethylene glycol) Films. ACS Appl. Polym. Mater. 2021, 3, 3446–3454. [Google Scholar] [CrossRef]
- Meyerbröker, N.; Zharnikov, M. Ultraflexible, freestanding nanomembranes based on polyethylene glycol. Adv. Mater. 2014, 26, 3328–3332. [Google Scholar] [CrossRef]
- Meyerbröker, N.; Zharnikov, M. Hydrogel Nanomembranes as Templates for Patterned Deposition of Nanoparticles on Arbitrary Substrates. ACS Appl. Mater. Interfaces 2014, 6, 14729–14735. [Google Scholar] [CrossRef]
- Khan, M.; Schuster, S.; Zharnikov, M. Giant electrical response of pristine and nanoparticle-loaded hydrogel nanomembranes to humidity variation. J. Phys. Chem. C 2015, 119, 14427–14433. [Google Scholar] [CrossRef]
- Small, M.K.; Vlassak, J.J.; Powell, S.F.; Daniels, B.J.; Nix, W.D. Accuracy and Reliability of Bulge Test Experiments. MRS Proc. 1993, 308, 159–164. [Google Scholar] [CrossRef]
- Paul, O.; Gaspar, J. Thin—Film characterization using the bulge test. In Advanced Micro and Nanosystems Vol. 6. Reliability of MEMS; Tabata, O., Tsuchiya, T., Eds.; WILEY-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2008; pp. 67–121. [Google Scholar]
- Zamprogno, P.; Thoma, G.; Cencen, V.; Ferrari, D.; Putz, B.; Michler, J.; Fantner, G.E.; Guenat, O.T. Mechanical Properties of Soft Biological Membranes for Organ-on-a-Chip Assessed by Bulge Test and AFM. ACS Biomater. Sci. Eng. 2021, 7, 2990–2997. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Stickle, W.E.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastian, J., Ed.; Perkin-Elmer Corp.: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Chen, K.-S.; Ou, K.-S. Handbook of Silicon Based MEMS Materials and Technologies; Tilli, M., Paulasto-Krockel, M., Petzold, M., Theuss, H., Motooka, T., Lindroos, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 398–412. [Google Scholar]
- Jiang, C.; Markutsya, S.; Pikus, Y.; Tsukruk, V.V. Freely suspended nanocomposite membranes as highly sensitive sensors. Nat. Mater. 2004, 3, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Beyer, A.; Gölzhäuser, A. Mechanical characterization of carbon nanomembranes from self-assembled monolayers. Beilstein J. Nanotechnol. 2011, 2, 826–833. [Google Scholar] [CrossRef] [Green Version]
- Datta, K.; Deotare, P.B. Optical Determination of Young’s Modulus of Nanoscale Organic Semiconductor Thin Films for Flexible Devices. ACS Appl. Nano Mater. 2019, 3, 992–1001. [Google Scholar] [CrossRef]
- Vlassak, J.J.; Nix, W.D. A new bulge test technique for the determination of Young’s modulus and Poisson’s ratio of thin films. J. Mater. Res. 1992, 7, 3242–3249. [Google Scholar] [CrossRef] [Green Version]
- Toda, M.; Miyake, K.; Chu, L.-Q.; Zakerin, M.; Förch, R.; Berger, R.; Itakura, A.N. Young’s modulus of plasma-polymerized allylamine films using micromechanical cantilever sensor and laser-based surface acoustic wave techniques. Plasma Process. Polym. 2018, 15, e1800083. [Google Scholar] [CrossRef]
- Ratner, M.; Castner, D. Electron spectroscopy for chemical analysis. In Surface Analysis—The Principal Techniques; Vickerman, J., Ed.; John Wiley & Sons: Chichester, NH, USA, 1997; pp. 43–98. [Google Scholar]
- Zharnikov, M.; Grunze, M. Modification of thiol-derived self-assembling monolayers by electron and X-ray irradiation: Scientific and lithographic aspects. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2002, 20, 1793. [Google Scholar] [CrossRef]
- Ballav, N.; Shaporenko, A.; Terfort, A.; Zharnikov, M. A Flexible Approach to the Fabrication of Chemical Gradients. Adv. Mater. 2007, 19, 998–1000. [Google Scholar] [CrossRef]
- Montague, M.; Ducker, R.E.; Chong, K.S.L.; Manning, R.J.; Rutten, F.J.M.; Davies, M.C.; Leggett, G.J. Fabrication of Biomolecular Nanostructures by Scanning Near-Field Photolithography of Oligo(ethylene glycol)-Terminated Self-Assembled Monolayers. Langmuir 2007, 23, 7328–7337. [Google Scholar] [CrossRef]
- Ballav, N.; Thomas, H.; Winkler, T.; Terfort, A.; Zharnikov, M. Making Protein Patterns by Writing in a Protein-Repelling Matrix. Angew. Chem. Int. Ed. 2009, 48, 5833–5836. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Terfort, A.; Zharnikov, M. Controlled modification of protein-repelling self-assembled monolayers by ultraviolet light: The effect of the wavelength. J. Phys. Chem. C 2012, 116, 9019–9028. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Zhu, X.-Y.; White, J.M. Photochemistry at adsorbate/metal interfaces. Surf. Sci. Rep. 1991, 13, 73–220. [Google Scholar] [CrossRef]
- Hasselbrink, E. Mechanisms in hhotochemistry on metal surfaces. Appl. Surf. Sci. 1994, 79–80, 34–40. [Google Scholar]
- Brewer, N.J.; Janusz, S.; Critchley, K.; Evans, A.S.D.; Leggett, G.J. Photooxidation of Self-Assembled Monolayers by Exposure to Light of Wavelength 254 nm: A Static SIMS Study. J. Phys. Chem. B 2005, 109, 11247–11256. [Google Scholar] [CrossRef]
- Correa-Puerta, J.; del Campo, V.; Henríquez, R.; Esaulov, V.A.; Hamoudi, H.; Flores, M.; Häberle, P. Unoccupied Interface and Molecular States in Thiol and Dithiol Monolayers. Langmuir 2017, 33, 12056–12064. [Google Scholar] [CrossRef]
- Zharnikov, M. Femtosecond Charge Transfer Dynamics in Monomolecular Films in the Context of Molecular Electronics. Acc. Chem. Res. 2020, 53, 2975–2984. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Liu, C. Recent Developments in Polymer MEMS. Adv. Mater. 2007, 19, 3783–3790. [Google Scholar] [CrossRef]
- Fan, S.; Meng, L.; Dan, L.; Zheng, W.; Wang, X. Polymer Microelectromechanical System-Integrated Flexible Sensors for Wearable Technologies. IEEE Sens. J. 2018, 19, 443–450. [Google Scholar] [CrossRef]
- Scherr, J.; Parey, K.; Klusch, N.; Murphy, B.J.; Balser, S.; Neuhaus, A.; Zickermann, V.; Kühlbrandt, W.; Terfort, A.; Rhinow, D. Self-Perforated Hydrogel Nanomembranes Facilitate Structural Analysis of Proteins by Electron Cryo-Microscopy. ACS Nano 2017, 11, 6467–6473. [Google Scholar] [CrossRef] [PubMed]
- Scherr, J.; Tang, Z.; Küllmer, M.; Balser, S.; Scholz, A.S.; Winter, A.; Parey, K.; Rittner, A.; Grininger, M.; Zickermann, V.; et al. Smart Molecular Nanosheets for Advanced Preparation of Biological Samples in Electron Cryo-Microscopy. ACS Nano 2020, 14, 9972–9978. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zharnikov, M. Elastic Properties of Poly(ethylene glycol) Nanomembranes and Respective Implications. Membranes 2022, 12, 509. https://doi.org/10.3390/membranes12050509
Zhao Z, Zharnikov M. Elastic Properties of Poly(ethylene glycol) Nanomembranes and Respective Implications. Membranes. 2022; 12(5):509. https://doi.org/10.3390/membranes12050509
Chicago/Turabian StyleZhao, Zhiyong, and Michael Zharnikov. 2022. "Elastic Properties of Poly(ethylene glycol) Nanomembranes and Respective Implications" Membranes 12, no. 5: 509. https://doi.org/10.3390/membranes12050509