MXene (Ti3C2Tx)/Cellulose Acetate Mixed-Matrix Membrane Enhances Fouling Resistance and Rejection in the Crossflow Filtration Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the MXene-CA Membrane
2.3. Characterization
2.4. Filtration Experiments
2.4.1. Dead-End Filtration
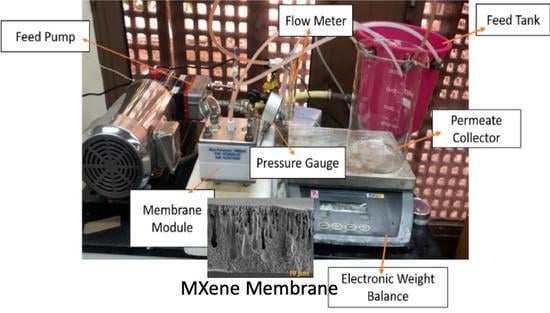
2.4.2. Crossflow Filtration
2.5. Antifouling Evaluation
3. Results and Discussion
3.1. Surface Characterization of MXene/CA Membranes
3.2. Hydrophilicity, Surface Area, and Morphology of CCAM and CCAM-X%
3.3. Membrane Separation Performance
3.3.1. Effect of Operating Pressure on Membrane Rejection
3.3.2. Effect of MXene Loading on Membrane Permeation and Rejection
3.4. Membrane Antifouling Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Romero-Vargas Castrillón, S.; Shaffer, D.L.; Ma, J.; Elimelech, M. In Situ Surface Chemical Modification of Thin-Film Composite Forward Osmosis Membranes for Enhanced Organic Fouling Resistance. Environ. Sci. Technol. 2013, 47, 12219. [Google Scholar] [CrossRef]
- Al-Najar, B.; Peters, C.D.; Albuflasa, H.; Hankins, N.P. Pressure and osmotically driven membrane processes: A review of the benefits and production of nano-enhanced membranes for desalination. Desalination 2020, 479, 114323. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Kwiecińska, A. Application of membrane processes in drinking water treatment–state of art. Desalination Water Treat.-DESALIN WATER TREAT 2011, 35, 164–184. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Zeolitic imidazolate framework-67/carboxylated graphene oxide nanosheets incorporated polyethersulfone hollow fiber membranes for removal of toxic heavy metals from contaminated water. Sep. Purif. Technol. 2020, 249, 117160. [Google Scholar] [CrossRef]
- Karimnezhad, H.; Navarchian, A.H.; Tavakoli Gheinani, T.; Zinadini, S. Incorporation of iron oxyhydroxide nanoparticles in polyacrylonitrile nanofiltration membrane for improving water permeability and antifouling property. React. Funct. Polym. 2019, 135, 77–93. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Wang, J.; Zhu, J.; Velizarov, S.; Van der Bruggen, B. Development and characterization of polyethersulfone-based nanofiltration membrane with stability to hydrogen peroxide. J. Membr. Sci. 2018, 550, 462–469. [Google Scholar] [CrossRef]
- Wei, C.; He, Z.; Lin, L.; Cheng, Q.; Huang, K.; Ma, S.; Chen, L. Negatively charged polyimide nanofiltration membranes with high selectivity and performance stability by optimization of synergistic imidization. J. Membr. Sci. 2018, 563, 752–761. [Google Scholar] [CrossRef]
- Shen, S.-S.; Chen, H.; Wang, R.-H.; Ji, W.; Zhang, Y.; Bai, R. Preparation of antifouling cellulose acetate membranes with good hydrophilic and oleophobic surface properties. Mater. Lett. 2019, 252, 1–4. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric antimicrobial membranes enabled by nanomaterials for water treatment. J. Membr. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Alosaimi, E.H.; Hassan, H.M.A.; Alsohaimi, I.H.; Chen, Q.; Melhi, S.; Younes, A.A.; El-Shwiniy, W.H. Fabrication of sulfonated polyethersulfone ultrafiltration membranes with an excellent antifouling performance by impregnating with polysulfopropyl acrylate coated ZnO nanoparticles. Environ. Technol. Innov. 2022, 25, 102210. [Google Scholar] [CrossRef]
- Beisl, S.; Monteiro, S.; Santos, R.; Figueiredo, A.S.; Sánchez-Loredo, M.G.; Lemos, M.A.; Lemos, F.; Minhalma, M.; de Pinho, M.N. Synthesis and bactericide activity of nanofiltration composite membranes–Cellulose acetate/silver nanoparticles and cellulose acetate/silver ion exchanged zeolites. Water Res. 2019, 149, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- García, M.G.; Marchese, J.; Ochoa, N.A. Effect of the particle size and particle agglomeration on composite membrane performance. J. Appl. Polym. Sci. 2010, 118, 2417–2424. [Google Scholar] [CrossRef]
- Duval, J.M.; Kemperman, A.J.B.; Folkers, B.; Mulder, M.H.V.; Desgrandchamps, G.; Smolders, C.A. Preparation of zeolite filled glassy polymer membranes. J. Appl. Polym. Sci. 1994, 54, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Casado-Coterillo, C. Mixed Matrix Membranes. Membranes 2019, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y. MXene materials for designing advanced separation membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasheed, P.A.; Gomez, T.; Azam, R.S.; Mahmoud, K.A. A fouling-resistant mixed-matrix nanofiltration membrane based on covalently cross-linked Ti3C2TX (MXene)/cellulose acetate. J. Membr. Sci. 2020, 607, 118139. [Google Scholar] [CrossRef]
- Alfahel, R.; Azzam, R.S.; Hafiz, M.; Hawari, A.H.; Pandey, R.P.; Mahmoud, K.A.; Hassan, M.K.; Elzatahry, A.A. Fabrication of fouling resistant Ti3C2Tx (MXene)/cellulose acetate nanocomposite membrane for forward osmosis application. J. Water Process Eng. 2020, 38, 101551. [Google Scholar] [CrossRef]
- Rasool, K.; Pandey, R.P.; Rasheed, P.A.; Buczek, S.; Gogotsi, Y.; Mahmoud, K.A. Water treatment and environmental remediation applications of two-dimensional metal carbides (MXenes). Mater. Today 2019, 30, 80–102. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Rasool, K.; Mahmoud, K.; Johnson, D.; I Helal, M.; Berdiyorov, G.; Gogotsi, Y. Efficient Antibacterial Membrane based on Two-Dimensional Ti3C2Tx (MXene) Nanosheets. Sci. Rep. 2017, 7, 1598. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Ren, C.E.; Zhao, M.-Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, X.; Bruggen, B. MXene based membrane for molecular separation. Environ. Sci. Nano 2020, 7, 1289–1304. [Google Scholar] [CrossRef]
- Azam, R.S. Enhancing The Fouling Resistance and Rejection of Cellulose Acetate [Ca]/Mxene [Ti3C2Tx] Nanocomposite Membranes; Qatar University: Doha, Qatar, 2021. [Google Scholar]
- Pandey, R.P.; Rasool, K.; Madhavan, V.E.; Aïssa, B.; Gogotsi, Y.; Mahmoud, K.A. Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2Tx) nanosheets. J. Mater. Chem. A 2018, 6, 3522–3533. [Google Scholar] [CrossRef]
- Singh, A.K.; Prakash, S.; Kulshrestha, V.; Shahi, V.K. Cross-linked Hybrid Nanofiltration Membrane with Antibiofouling Properties and Self-Assembled Layered Morphology. ACS Appl. Mater. Interfaces 2012, 4, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Teo, J.; Chung, T.-S. Cellulose acetate nanofiltration hollow fiber membranes for forward osmosis processes. J. Membr. Sci. 2010, 355, 36–44. [Google Scholar] [CrossRef]
- Vatanpour, V.; Shockravi, A.; Zarrabi, H.; Nikjavan, Z.; Javadi, A. Fabrication and characterization of anti-fouling and anti-bacterial Ag-loaded graphene oxide/polyethersulfone mixed matrix membrane. J. Ind. Eng. Chem. 2015, 30, 342–352. [Google Scholar] [CrossRef]
- Changa, X.; Wanga, Z.; Quana, S.; Xua, Y.; Jianga, Z.; Shaoa, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Shi, B.; Guo, W.; Jaroniec, M.; Qiao, S.-Z. A Regularly Channeled Lamellar Membrane for Unparalleled Water and Organics Permeation. Angew. Chem. Int. Ed. 2018, 57, 6814–6818. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.; Sadasivuni, K.K.; Mun, S.; Kim, J. Flexible cellulose acetate/graphene blueprints for vibrotactile actuator. RSC Adv. 2015, 5, 34432–34438. [Google Scholar] [CrossRef]
- Liu, R.; Li, W. High-Thermal-Stability and High-Thermal-Conductivity Ti3C2Tx MXene/Poly(vinyl alcohol) (PVA) Composites. ACS Omega 2018, 3, 2609–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.; Chen, W.; Xu, H.; Yang, W.; Kong, Q.; Wang, A.; Ding, M.; Shang, J. Fabrication of a Novel Antifouling Polysulfone Membrane with in Situ Embedment of Mxene Nanosheets. Int. J. Environ. Res. Public Health 2019, 16, 4659. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; De, S. Adsorptive removal of arsenic from groundwater using a novel high flux polyacrylonitrile (PAN)–laterite mixed matrix ultrafiltration membrane. Environ. Sci. Water Res. Technol. 2015, 1, 227–243. [Google Scholar] [CrossRef]
- Han, R.; Xie, Y.; Ma, X. Crosslinked P84 copolyimide/MXene mixed matrix membrane with excellent solvent resistance and permselectivity. Chin. J. Chem. Eng. 2019, 27, 877–883. [Google Scholar] [CrossRef]
- Alberto Figoli, J.H. Sacide Alsoy Altinkaya, Jochen Bundschuh. Application of Nanotechnology in Membranes for Water Treatment; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Ryan, B.J.; Poduska, K.M. Roughness effects on contact angle measurements. Am. J. Phys. 2008, 76, 1074–1077. [Google Scholar] [CrossRef] [Green Version]
- Singh, R. (Ed.) Chapter 1-Introduction to Membrane Technology. In Membrane Technology and Engineering for Water Purification, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2015; pp. 1–80. [Google Scholar]
- Sun, W.; Liu, J.; Chu, H.; Dong, B. Pretreatment and Membrane Hydrophilic Modification to Reduce Membrane Fouling. Membranes 2013, 3, 226–241. [Google Scholar] [CrossRef]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]
- Wang, X.; Ba, X.; Cui, N.; Ma, Z.; Wang, L.; Wang, Z.; Gao, X. Preparation, characterisation, and desalination performance study of cellulose acetate membranes with MIL-53(Fe) additive. J. Membr. Sci. 2019, 590, 117057. [Google Scholar] [CrossRef]
- Alosaimi, E.H.; Hotan Alsohaimi, I.; Hassan, H.M.A.; Chen, Q.; Melhi, S.; Abdelaziz Younes, A. Towards superior permeability and antifouling performance of sulfonated polyethersulfone ultrafiltration membranes modified with sulfopropyl methacrylate functionalized SBA-15. Chin. J. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Alhoshan, M.; Alam, J.; Dass, L.A.; Al-Homaidi, N. Fabrication of Polysulfone/ZnO Membrane: Influence of ZnO Nanoparticles on Membrane Characteristics. Adv. Polym. Technol. 2013, 32, 1–7. [Google Scholar] [CrossRef]
- Ko, K.; Yu, Y.; Kim, M.-J.; Kweon, J.; Chung, H. Improvement in fouling resistance of silver-graphene oxide coated polyvinylidene fluoride membrane prepared by pressurized filtration. Sep. Purif. Technol. 2018, 194, 161–169. [Google Scholar] [CrossRef]
- Ní Mhurchú, J. Dead-End and Crossflow Microfiltration of Yeast and Bentonite Suspensions: Experimental and Modelling Studies Incorporating the Use of Artificial Neural Networks; Dublin City University: Dublin, Ireland, 2008. [Google Scholar]
- Wang, Q.; Zhang, S.; Ji, X.; Ran, F. High rejection performance ultrafiltration membrane with ultrathin dense layer fabricated by the movement and dissolution of metal–organic frameworks. New J. Chem. 2020, 44, 13745–13754. [Google Scholar] [CrossRef]
- Al-Shaeli, M.; Smith, S.J.D.; Jiang, S.; Wang, H.; Zhang, K.; Ladewig, B.P. Long-term stable metal organic framework (MOF) based mixed matrix membranes for ultrafiltration. J. Membr. Sci. 2021, 635, 119339. [Google Scholar] [CrossRef]
- Kusumocahyo, S.; Ambani, S.; Marceline, S. Improved permeate flux and rejection of ultrafiltration membranes prepared from polyethylene terephthalate (PET) bottle waste. Sustain. Environ. Res. 2021, 31, 19. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, H.; Wang, C.; Zhang, S.; Zhang, L.; Liu, X.; Liu, F.; Zhu, X.; Rohani, S.; Ching, C.; et al. A novel PVDF/PFSA-g-GO ultrafiltration membrane with enhanced permeation and antifouling performances. Sep. Purif. Technol. 2020, 233, 116038. [Google Scholar] [CrossRef]
- Kassa, S.T.; Hu, C.C.; Keshebo, D.L.; Belle Marie Ang, M.; Lai, J.Y.; Chu, J.P. Surface modification of high-rejection ultrafiltration membrane with antifouling capability using activated oxygen treatment and metallic glass deposition. Appl. Surf. Sci. 2020, 529, 147131. [Google Scholar] [CrossRef]
- Vetrivel, S.; Sri Abirami Saraswathi, M.; Rana, D.; Divya, K.; Nagendran, A. Cellulose acetate composite membranes tailored with exfoliated tungsten disulfide nanosheets: Permeation characteristics and antifouling ability. Int. J. Biol. Macromol. 2018, 115, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Rehan, Z.A.; Khan, S.A.; Akhtar, K.; Khan, M.A.; Khan, M.I.; Rashid, M.I.; Asiri, A.M.; Khan, S.B. Antibacterial PES-CA-Ag2O nanocomposite supported Cu nanoparticles membrane toward ultrafiltration, BSA rejection and reduction of nitrophenol. J. Mol. Liq. 2017, 230, 616–624. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Hao, M.; Huang, C.; Xue, Z.; Mu, T. Preparation and characterization of regenerated cellulose from ionic liquid using different methods. Carbohydr. Polym. 2015, 117, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, S.; Alrubaye, J.; Albayati, T. Removal of Methyl Green Dye from simulated waste water using Hollow Fiber Ultrafiltration Membrane. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 052020. [Google Scholar] [CrossRef]
- Dasgupta, J.; Chakraborty, S.; Sikder, J.; Kumar, R.; Pal, D.; Curcio, S.; Drioli, E. The effects of thermally stable titanium silicon oxide nanoparticles on structure and performance of cellulose acetate ultrafiltration membranes. Sep. Purif. Technol. 2014, 133, 55–68. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Development of novel SiO2–GO nanohybrid/polysulfone membrane with enhanced performance. J. Membr. Sci. 2014, 451, 94–102. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Fang, F.; Deng, X.; Ma, S. Astragaloside IV attenuates cognitive impairments induced by transient cerebral ischemia and reperfusion in mice via anti-inflammatory mechanisms. Neurosci. Lett. 2017, 639, 114–119. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, S. Fabrication of a novel cyanoethyl cellulose substrate for thin-film composite forward osmosis membrane. Blue-Green Syst. 2019, 1, 18–32. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R.; Mohammad, A. Enhancing Morphology and Separation Performance of Polyamide 6,6 Membranes By Minimal Incorporation of Silver Decorated Graphene Oxide Nanoparticles. Sci. Rep. 2019, 9, 1216. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; McGlade, D.; Ulbricht, M.; Lawler, J. Quaternized polysulfone and graphene oxide nanosheet derived low fouling novel positively charged hybrid ultrafiltration membranes for protein separation. RSC Adv. 2015, 5, 51208–51219. [Google Scholar] [CrossRef] [Green Version]
- Moradi, G.; Zinadini, S. A high flux graphene oxide nanoparticles embedded in PAN nanofiber microfiltration membrane for water treatment applications with improved anti-fouling performance. Iran. Polym. J. 2020, 29, 827–840. [Google Scholar] [CrossRef]







| Membrane | CCAM-0% | CCAM-10% |
|---|---|---|
| Average roughness (Ra (nm)) | 22.50 | 47.40 |
| Root square roughness (Rq (nm)) | 28.40 | 60.20 |
| Membrane | Specific Surface Area (m2/g) | Mean Pore Diameter (nm) | |
|---|---|---|---|
| CCAM-0% | 44.27 | 12.83 | 0.284 |
| CCAM-10% | 124.3 | 1.910 | 0.781 |
| Membrane | Rejected Dye/Protein | Rejection (%), Dead-End | Rejection (%), Crossflow |
|---|---|---|---|
| CCAM-0% | MG | 28.91 | 49.70 |
| BSA | 73.00 | 82.75 | |
| CCAM-8% | MG | 79.90 | 80.33 |
| BSA | 90.80 | 97.23 | |
| CCAM-10% | MG | 92.13 | 96.60 |
| BSA | 97.97 | 99.51 |
| Membrane | Dye/Protein | Water Flux (L m−2h−1bar−1) | Rejection (%) | Reference |
|---|---|---|---|---|
| M-PES/ZIF-67 | BSA | ~56.00 | 98.00 | [48] |
| MOFs UiO-66 NH2-PES-MMM | BSA | 300.0 | 95.00 | [49] |
| PET-PEG3 | BSA | ~12.00 | 94.00 | [50] |
| PVDF/PFSA | BSA | ~461.0 | 88.00 | [51] |
| TFMGs/W-PSF10 | BSA | ~322.0 | 99.90 | [52] |
| CA/E-WS2(1 wt.%) | BSA | ~107.0 | ~97.00 | [53] |
| PES-CA | BSA | ~63.00 | ~ 85.00 | [54] |
| PES-CA-Ag2O | ~93.00 | ~89.00 | ||
| ZrO2/BCM | BSA | ~322.0 | ~91.00 | [55] |
| 18 wt.% PVC Hollow fiber | MG | ~32.00 | 75.20 | [56] |
| CA/TiSiO4 (20 wt.%) | BSA | 134.0 | 98.80 | [57] |
| PSF/MXene | BSA | 306.0 | 98.00 | [34] |
| PSF/SiO2GO | BSA | 376.0 | 98.00 | [58] |
| PVDF/GO | BSA | 243.0 | ~77.00 | [59] |
| ZCA | BSA | ~137.0 | ~98.00 | [60] |
| MXene (Ti3C2Tx) | MG | 118.0 | 94.00 | [26] |
| BSA | 100.0 | |||
| 21% Ag at MXene | MG | 420.0 | 92.00 | |
| BSA | 100.0 | |||
| CCAM-10% | MG | 522.3 | 96.60 | This Work |
| BSA | 99.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azam, R.S.; Almasri, D.A.; Alfahel, R.; Hawari, A.H.; Hassan, M.K.; Elzatahry, A.A.; Mahmoud, K.A. MXene (Ti3C2Tx)/Cellulose Acetate Mixed-Matrix Membrane Enhances Fouling Resistance and Rejection in the Crossflow Filtration Process. Membranes 2022, 12, 406. https://doi.org/10.3390/membranes12040406
Azam RS, Almasri DA, Alfahel R, Hawari AH, Hassan MK, Elzatahry AA, Mahmoud KA. MXene (Ti3C2Tx)/Cellulose Acetate Mixed-Matrix Membrane Enhances Fouling Resistance and Rejection in the Crossflow Filtration Process. Membranes. 2022; 12(4):406. https://doi.org/10.3390/membranes12040406
Chicago/Turabian StyleAzam, Reem S., Dema A. Almasri, Radwan Alfahel, Alaa H. Hawari, Mohammad K. Hassan, Ahmed A. Elzatahry, and Khaled A. Mahmoud. 2022. "MXene (Ti3C2Tx)/Cellulose Acetate Mixed-Matrix Membrane Enhances Fouling Resistance and Rejection in the Crossflow Filtration Process" Membranes 12, no. 4: 406. https://doi.org/10.3390/membranes12040406