A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment
Abstract
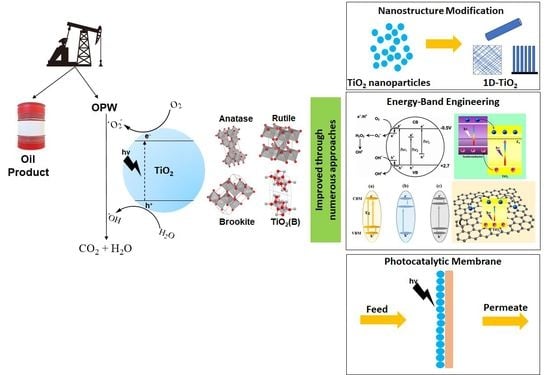
:1. Introduction
2. Modification Approaches of TiO2 Photocatalyst
2.1. Construction of TiO2 Nanostructures Form
2.2. Energy Band Engineering for TiO2
2.3. TiO2-Based Photocatalytic Membrane (PM)
3. Challenges and Future Development
- (1)
- There is not much research focused on treating OPW directly, as well as optimization of photodegradation performance, taking into account numerous aspects, such as pH condition, pollutant concentration, organic species variety, and light source and intensity, which affect the quality and sustainability of those technologies themselves. Apart from the environmental consequences of high organic effluent concentration in OPW, the amount of energy required has a significant impact on the photocatalysis process. This is one of the reasons why solar photocatalysis has recieved so much interest recently.
- (2)
- A life cycle assessment (LCA) of those technologies that are needed for large scale application, especially the treatment of OPW. LCA is one of the most important tools for identifying the environmental effects of a process, as well as its feasibility and costs. Although numerous studies have applied for photodegradation, there is still a lack of understanding about their end of life.
- (3)
- In terms of industrial scale feasibility, whether the photocatalytic process should be employed as a pre-treatment or as an independent treatment for OPW degradation become major constraints.
- Determining the most appropriate semiconductor and/or doping element that boosts TiO2 photocatalytic performance and stability, which is in agreement with physicochemical properties, such as the active surface sites, morphology, optical, and electron transfer properties. As a result, both teoritical and experimental aspects can be supported by this approach.
- Applying the photodegradation process of the OPW model solution using common organic pollutant species, such as phenol, BTEX, etc. Then, more in-depth thermodynamic and kinetic analyses are also possible.
- Conceptualizing the process flow design of OPW photodegradation process using high novelty TiO2-based PM. This needs to be considered in terms of techno-economic and LCA parameters so that the sustainability of TiO2-based PM can be optimized for long-term industrial applications.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alias, N.H.; Jaafar, J.; Samitsu, S.; Matsuura, T.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Othman, N.H.; Abdullah, N.; Paiman, S.H.; et al. Photocatalytic nanofiber-coated alumina hollow fiber membranes for highly efficient oilfield produced water treatment. Chem. Eng. J. 2019, 360, 1437–1446. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-mechanistic aspects and process chemistry perspectives. Chem. Eng. J. Adv. 2020, 4, 100049. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Jing, L.; Chen, B.; Zhang, B.; Zheng, J.; Liu, B. Naphthalene degradation in seawater by UV irradiation: The effects of fluence rate, salinity, temperature and initial concentration. Mar. Pollut. Bull. 2014, 81, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.C.; Ferraz, N.P.; Perpetuo, E.A.; Asencios, Y.J.O. Oil produced water treatment using advanced oxidative processes: Heterogeneous-photocatalysis and photo-fenton. J. Sediment. Environ. 2019, 4, 99–107. [Google Scholar] [CrossRef]
- Zioui, D.; Salazar, H.; Aoudjit, L.; Martins, P.M. Photocatalytic polymeric nanocomposite membrane towards oily wastewater. Preprints 2019, 2019040060. [Google Scholar] [CrossRef]
- Afzal, T.; Isa, M.H.; Ul Mustafa, M.R. Removal of organic pollutants from produced water using Fenton oxidation. E3S Web Conf. 2018, 34, 02035. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yang, P. Review on Physicochemical, Chemical, and Biological Processes for Pharmaceutical Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012185. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, B. Oily Wastewater Treatment by Nano-TiO2-Induced Photocatalysis: Seeking more efficient and feasible solutions. IEEE Nanotechnol. Mag. 2017, 11, 4–15. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Kanan, S.; Moyet, M.A.; Arthur, R.B.; Patterson, H.H. Recent advances on TiO2-based photocatalysts toward the degradation of pesticides and major organic pollutants from water bodies. Catal. Rev. Sci. Eng. 2020, 62, 1–65. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H. Comparison of azo dye degradation efficiency using UV/single semiconductor and UV/coupled semiconductor systems. Chemosphere 2004, 57, 601–608. [Google Scholar] [CrossRef]
- Augugliaro, V.; Palmisano, L.; Sclafani, A.; Minero, C.; Pelizzetti, E. Photocatalytic Degradation of Phenol in Aqueous Titanium Dioxide Dispersions. Toxicol. Environ. Chem. 1988, 16, 89–109. [Google Scholar] [CrossRef]
- Okamoto, K.; Yamamoto, Y.; Tanaka, H.; Tanaka, M.; Itaya, A. Heterogeneous Photocatalytic Decomposition of Phenol over TiO2 Powder. Bull. Chem. Soc. Jpn. 1985, 58, 2015–2022. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Chen, B.; Zhang, B.Y.; Jing, L.; Zhang, H.; Lee, K. Photocatalytic Degradation of Polycyclic Aromatic Hydrocarbons in Offshore Produced Water: Effects of Water Matrix. J. Environ. Eng. 2016, 142, 04016054. [Google Scholar] [CrossRef]
- Li, G.; An, T.; Chen, J.; Sheng, G.; Fu, J.; Chen, F.; Zhang, S.; Zhao, H. Photoelectrocatalytic decontamination of oilfield produced wastewater containing refractory organic pollutants in the presence of high concentration of chloride ions. J. Hazard. Mater. 2006, 138, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Mølhave, K.; Sun, H. Engineering the surface/interface structures of titanium dioxide micro and nano architectures towards environmental and electrochemical applications. Nanomaterials 2017, 7, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; De Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Marchand, R.; Brohan, L.; Tournoux, M. TiO2(B) A New Form of Titanium Dioxide and the Potassium Octatitanate K2Ti8O17. Mater. Res. Bull. 1980, 15, 1129–1133. [Google Scholar] [CrossRef]
- Pawar, M.; Sendoǧdular, S.T.; Gouma, P. A brief overview of TiO2 photocatalyst for organic dye remediation: Case study of reaction mechanisms involved in Ce-TiO2 photocatalysts system. J. Nanomater. 2018, 2018, 5953609. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [Green Version]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Ding, H.; Feng, J.; Hao, Q.; Sun, S.; Ao, W.; Chen, D. Highly Performance Core-Shell TiO2(B)/anatase Homojunction Nanobelts with Active Cobalt phosphide Cocatalyst for Hydrogen Production. Sci. Rep. 2017, 7, 14594. [Google Scholar] [CrossRef]
- Kamaludin, R.; Othman, M.H.D.; Kadir, S.H.S.A.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. Visible-Light-Driven Photocatalytic N-Doped TiO2 for Degradation of Bisphenol A (BPA) and Reactive Black 5 (RB5) Dye. Water. Air. Soil Pollut. 2018, 229, 363. [Google Scholar] [CrossRef]
- Luo, W.; Taleb, A. Large-scale synthesis route of TiO2 nanomaterials with controlled morphologies using hydrothermal method and TiO2 aggregates as precursor. Nanomaterials 2021, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Cassaignon, S.; Koelsch, M.; Jolivet, J.P. From TiCl3 to TiO2 nanoparticles (anatase, brookite and rutile): Thermohydrolysis and oxidation in aqueous medium. J. Phys. Chem. Solids 2007, 68, 695–700. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Okoh, O.; Mungondori, H.; Taziwa, R.; Zinya, S. Synthetic Methods for Titanium Dioxide Nanoparticles: A Review. In Titanium Dioxide—Material for a Sustainable Environment; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Mironyuk, I.F.; Soltys, L.M.; Tatarchuk, T.R.; Savka, K.O. Methods of titanium dioxide synthesis (review). Phys. Chem. Solid State 2020, 21, 462–477. [Google Scholar] [CrossRef]
- Joni, I.M.; Nulhakim, L.; Vanitha, M.; Panatarani, C. Characteristics of TiO2 particles prepared by simple solution method using TiCl3 precursor. J. Phys. Conf. Ser. 2018, 1080, 012006. [Google Scholar] [CrossRef]
- Karkare, M.M. Choice of precursor not affecting the size of anatase TiO2 nanoparticles but affecting morphology under broader view. Int. Nano Lett. 2014, 4, 111. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Mukherjee, S.; Ghosh, C.K.; Maitra, S.; Technology, C. Influence of precursor type on structural, morphological, dielectric and magnetic properties of TiO2 nanoparticles (Influência do tipo precursor nas propriedades estruturais, morfológicas). Ceramica 2017, 63, 549–556. [Google Scholar] [CrossRef]
- Sagadevan, S.; Imteyaz, S.; Murugan, B.; Anita Lett, J.; Sridewi, N.; Weldegebrieal, G.K.; Fatimah, I.; Oh, W.C. A comprehensive review on green synthesis of titanium dioxide nanoparticles and their diverse biomedical applications. Green Process. Synth. 2022, 11, 44–63. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; ur Rehman, M.Z.; Adrees, M.; Rizwan, M.; Ali, S.; Ahmad, S.; Tasleem, S. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2021, 212, 111978. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Meng, X.; Wang, Z.; Zhou, J.; Tang, H. One-step electrospinning synthesis of TiO2/g-C3N4 nanofibers with enhanced photocatalytic properties. Appl. Surf. Sci. 2018, 430, 253–262. [Google Scholar] [CrossRef]
- Wang, T.; Xu, J.; Zhang, Z.; Bian, H.; Xiao, H.; Sun, T. g-C3N4 composited TiO2 nanofibers were prepared by high voltage electrostatic spinning to improve photocatalytic efficiency. J. Mater. Sci. Mater. Electron. 2021, 32, 1178–1186. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Minero, C.; Vione, D. A quantitative evalution of the photocatalytic performance of TiO2 slurries. Appl. Catal. B Environ. 2006, 67, 257–269. [Google Scholar] [CrossRef]
- Liu, B.; Chen, B.; Zhang, B.; Zheng, J.; Liang, J. Toxicity and Biodegradability Study on Enhanced Photocatalytic Oxidation of Polycyclic Aromatic Hydrocarbons in Offshore Produced Water. In Proceedings of the Canadian Society of Civil Engineering Annual General Conference, Vancouver, BC, Canada, 31 May–3 June 2017; pp. 1–2. [Google Scholar]
- Reghunath, S.; Pinheiro, D.; Sunaja Devi, K.R. A review of hierarchical nanostructures of TiO2: Advances and applications. Appl. Surf. Sci. Adv. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Feng, T.; Feng, G.S.; Yan, L.; Pan, J.H. One-dimensional nanostructured TiO2 for photocatalytic degradation of organic pollutants in wastewater. Int. J. Photoenergy 2014, 2014, 563879. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Wen, W.; Jin, Q.; Xiang, X.B.; Wu, J.M. TiO2 nanotrees for the photocatalytic and photoelectrocatalytic phenol degradation. New J. Chem. 2019, 43, 11050–11056. [Google Scholar] [CrossRef]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic membranes in photocatalytic membrane reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Lin, T.S. Enhancement of visible-light photocatalytic efficiency of TiO2 nanopowder by anatase/rutile dual phase formation. Appl. Sci. 2020, 10, 6353. [Google Scholar] [CrossRef]
- Yang, H. A short review on heterojunction photocatalysts: Carrier transfer behavior and photocatalytic mechanisms. Mater. Res. Bull. 2021, 142, 111406. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Liu, X.; Yu, M.; Wang, C.; Li, J. The highly efficient and stable Cu, Co, Zn-porphyrin–TiO2 photocatalysts with heterojunction by using fashioned one-step method. Dye. Pigment. 2017, 136, 648–656. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, X.; Wang, D.; Dong, W.; Dong, C.; Zhang, Y.; Lin, T.; Huang, F. Tunable Synthesis of Colorful Nitrogen-Doped Titanium Oxide and Its Application in Energy Storage. ACS Appl. Energy Mater. 2018, 1, 876–882. [Google Scholar] [CrossRef]
- Niu, P.; Wu, T.; Wen, L.; Tan, J.; Yang, Y.; Zheng, S.; Liang, Y.; Li, F.; Irvine, J.T.S.; Liu, G.; et al. Substitutional Carbon-Modified Anatase TiO2 Decahedral Plates Directly Derived from Titanium Oxalate Crystals via Topotactic Transition. Adv. Mater. 2018, 30, 1705999. [Google Scholar] [CrossRef] [Green Version]
- Hachiya, A.; Takata, S.; Komuro, Y.; Matsumoto, Y. Effects of V-ion doping on the photoelectrochemical properties of epitaxial TiO2(110) thin films on Nb-doped TiO2(110) single crystals. J. Phys. Chem. C 2012, 116, 16951–16956. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Q. Mechanism of higher photocatalytic activity of anatase TiO2 doped with nitrogen under visible-light irradiation from density functional theory calculation. J. Phys. D Appl. Phys. 2008, 41, 025105. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Irie, H.; Watanabe, Y.; Hashimoto, K. Nitrogen-concentration dependence on photocatalytic activity of TiO2-xNx powders. J. Phys. Chem. B 2003, 107, 5483–5486. [Google Scholar] [CrossRef]
- Ihara, T.; Miyoshi, M.; Iriyama, Y.; Matsumoto, O.; Sugihara, S. Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Appl. Catal. B Environ. 2003, 42, 403–409. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review. Recent Patents Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Wang, G.; Zhuo, X.; Wang, Y. Photocatalytic degradation of polyacrylamide in oilfield sewage by nano-sized TiO2 doped with W Ion. MATEC Web Conf. 2016, 39, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.; Verma, P.; Sharma, H.; Tripathy, S.; Saini, V.K. Photodegradation of 4-nitrophenol over B-doped TiO2 nanostructure: Effect of dopant concentration, kinetics, and mechanism. Environ. Sci. Pollut. Res. 2020, 27, 10966–10980. [Google Scholar] [CrossRef]
- Lee, M.S.; Hong, S.S.; Mohseni, M. Synthesis of photocatalytic nanosized TiO2-Ag particles with sol-gel method using reduction agent. J. Mol. Catal. A Chem. 2005, 242, 135–140. [Google Scholar] [CrossRef]
- Zaleska, A.; Sobczak, J.W.; Grabowska, E.; Hupka, J. Preparation and photocatalytic activity of boron-modified TiO2 under UV and visible light. Appl. Catal. B Environ. 2008, 78, 92–100. [Google Scholar] [CrossRef]
- Feilizadeh, M.; Delparish, A.; Toufigh Bararpour, S.; Najafabadi, H.A.; Zakeri, S.M.E.; Vossoughi, M. Photocatalytic removal of 2-nitrophenol using silver and sulfur co-doped TiO2 under natural solar light. Water Sci. Technol. 2015, 72, 339–346. [Google Scholar] [CrossRef]
- Laokiat, L.; Khemthong, P.; Grisdanurak, N.; Sreearunothai, P.; Pattanasiriwisawa, W.; Klysubun, W. Photocatalytic degradation of benzene, toluene, ethylbenzene, and xylene (BTEX) using transition metal-doped titanium dioxide immobilized on fiberglass cloth. Korean J. Chem. Eng. 2012, 29, 377–383. [Google Scholar] [CrossRef]
- Binas, V.; Stefanopoulos, V.; Kiriakidis, G.; Papagiannakopoulos, P. Photocatalytic oxidation of gaseous benzene, toluene and xylene under UV and visible irradiation over Mn-doped TiO2 nanoparticles. J. Mater. 2019, 5, 56–65. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, J.H. Sonochemical synthesis of Ce-doped TiO2 nanostructure: A visible-light-driven photocatalyst for degradation of toluene and O-Xylene. Materials 2019, 12, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Mohd Adnan, M.A.; Muhd Julkapli, N.; Amir, M.N.I.; Maamor, A. Effect on different TiO2 photocatalyst supports on photodecolorization of synthetic dyes: A review. Int. J. Environ. Sci. Technol. 2019, 16, 547–566. [Google Scholar] [CrossRef]
- Sun, C.; Xu, Q.; Xie, Y.; Ling, Y.; Hou, Y. Designed synthesis of anatase-TiO2(B) biphase nanowire/ZnO nanoparticle heterojunction for enhanced photocatalysis. J. Mater. Chem. A 2018, 6, 8289–8298. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Zheng, Z.; Yuan, Y.; Zhao, J.C.; Waclawik, E.R.; Ke, X.; Zhu, H. An efficient photocatalyst structure: TiO2(B) nanofibers with a shell of anatase nanocrystals. J. Am. Chem. Soc. 2009, 131, 17885–17893. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, W.; Pan, W. Enhanced photocatalytic activity of electrospun TiO2 nanofibers with optimal anatase/rutile ratio. J. Am. Ceram. Soc. 2011, 94, 3184–3187. [Google Scholar] [CrossRef]
- Apopei, P.; Catrinescu, C.; Teodosiu, C.; Royer, S. Mixed-phase TiO2 photocatalysts: Crystalline phase isolation and reconstruction, characterization and photocatalytic activity in the oxidation of 4-chlorophenol from aqueous effluents. Appl. Catal. B Environ. 2014, 160–161, 374–382. [Google Scholar] [CrossRef]
- Ai, M.; Qin, W.; Xia, T.; Ye, Y.; Chen, X.; Zhang, P. Photocatalytic degradation of 2,4-dichlorophenol by TiO2 intercalated talc nanocomposite. Int. J. Photoenergy 2019, 2019, 1540271. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Teng, C.; Wang, S.; Min, Q. Recent Advances in TiO2-Based Heterojunctions for Photocatalytic CO2 Reduction With Water Oxidation: A Review. Front. Chem. 2021, 9, 637501. [Google Scholar] [CrossRef]
- Akhlaghian, F.; Najafi, A. CuO/WO3/TiO2 photocatalyst for degradation of phenol wastewater. Sci. Iran. 2018, 25, 3345–3353. [Google Scholar] [CrossRef] [Green Version]
- Rangkooy, H.A.; Ghaedi, H.; Jahani, F. Removal of xylene vapor pollutant from the air using new hybrid substrates of TiO2-WO3 nanoparticles immobilized on the ZSM-5 zeolite under UV radiation at ambient temperature: Experimental towards modeling. J. Environ. Chem. Eng. 2019, 7, 103247. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Zu, H.; Zhang, Z.; Han, J. Construction of TiO2/CdS heterojunction photocatslysts with enhanced visible light activity. Appl. Surf. Sci. 2018, 455, 729–735. [Google Scholar] [CrossRef]
- Kumari, M.L.A.; Devi, L.G.; Maia, G.; Chen, T.W.; Al-Zaqri, N.; Ali, M.A. Mechanochemical synthesis of ternary heterojunctions TiO2(A)/TiO2(R)/ZnO and TiO2(A)/TiO2(R)/SnO2 for effective charge separation in semiconductor photocatalysis: A comparative study. Environ. Res. 2022, 203, 111841. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, H.; Luo, X.; Li, M.; Dai, M.; Chen, Q.; Song, H. Enhanced photocatalytic properties of CeO2/TiO2 heterostructures for phenol degradation. Colloids Interface Sci. Commun. 2021, 44, 100476. [Google Scholar] [CrossRef]
- Telegang Chekem, C.; Goetz, V.; Richardson, Y.; Plantard, G.; Blin, J. Modelling of adsorption/photodegradation phenomena on AC-TiO2 composite catalysts for water treatment detoxification. Catal. Today 2019, 328, 183–188. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Zhang, W.; Zhu, Z.; Ma, J. Study on photocatalytic mechanism of Fe3+-TiO2-zeolite for oilfield wastewater treatment. In Proceedings of the 2010 International Conference on Digital Manufacturing & Automation, Changcha, China, 18–20 December 2010; Volume 2, pp. 526–529. [Google Scholar] [CrossRef]
- Syed, M.A.; Mauriya, A.K.; Shaik, F. Investigation of epoxy resin/nano-TiO2 composites in photocatalytic degradation of organics present in oil-produced water. J. Environ. Anal. Chem. 2020, 1–17. [Google Scholar] [CrossRef]
- Sökmen, M.; Özkan, A. Decolourising textile wastewater with modified titania: The effects of inorganic anions on the photocatalysis. J. Photochem. Photobiol. A Chem. 2002, 147, 77–81. [Google Scholar] [CrossRef]
- Andreozzi, M.; Álvarez, M.G.; Contreras, S.; Medina, F.; Clarizia, L.; Vitiello, G.; Llorca, J.; Marotta, R. Treatment of saline produced water through photocatalysis using rGO-TiO2 nanocomposites. Catal. Today 2018, 315, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Rongan, H.; Haijuan, L.; Huimin, L.; Difa, X.; Liuyang, Z. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance. J. Mater. Sci. Technol. 2020, 52, 145–151. [Google Scholar] [CrossRef]
- Jo, W.K.; Lee, J.Y.; Chun, H.H. Titania nanotubes grown on carbon fibers for photocatalytic decomposition of gas-phase aromatic pollutants. Materials 2014, 7, 1801–1813. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Du, L.; Gao, W.; Li, J.; Tsona, N.T.; Zhang, X.; Hu, X.; Wang, W.; Liu, H. Enhanced photocatalytic performance of PdO-loaded heterostructured nanobelts to degrade phenol. Chemosphere 2021, 276, 130266. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of produced water with photocatalysis: Recent advances, affecting factors and future research prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Chen, L.; Xu, P.; Wang, H. Photocatalytic membrane reactors for produced water treatment and reuse: Fundamentals, affecting factors, rational design, and evaluation metrics. J. Hazard. Mater. 2022, 424, 127493. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Yousefi Kebria, D.; Qaderi, F. Effect of biosurfactant as a novel draw solution on photocatalytic treatment and desalination of produced water by different forward osmosis membranes. Water Sci. Technol. Water Supply 2020, 20, 240–250. [Google Scholar] [CrossRef]
- Rawindran, H.; Lim, J.W.; Goh, P.S.; Subramaniam, M.N.; Ismail, A.F.; Radi bin Nik M Daud, N.M.; Rezaei-Dasht Arzhandi, M. Simultaneous separation and degradation of surfactants laden in produced water using PVDF/TiO2 photocatalytic membrane. J. Clean. Prod. 2019, 221, 490–501. [Google Scholar] [CrossRef]
- Veréb, G.; Kassai, P.; Nascimben Santos, E.; Arthanareeswaran, G.; Hodúr, C.; László, Z. Intensification of the ultrafiltration of real oil-contaminated (produced) water with pre-ozonation and/or with TiO2, TiO2/CNT nanomaterial-coated membrane surfaces. Environ. Sci. Pollut. Res. 2020, 27, 22195–22205. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Yang, G.; Cheng, C.; Huang, C.; Xu, H.; Ke, Q. Hierarchically structured TiO2/PAN nanofibrous membranes for high-efficiency air filtration and toluene degradation. J. Colloid Interface Sci. 2017, 507, 386–396. [Google Scholar] [CrossRef]
- Labuto, G.; Sanches, S.; Crespo, J.G.; Pereira, V.J.; Huertas, R.M. Stability of polymeric membranes to UV exposure before and after coating with TiO2 nanoparticles. Polymers 2022, 14, 124. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Bai, H.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res. 2012, 46, 1101–1112. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Yan, J.; Yu, J.; Sun, G.; Ding, B. Soft Zr-doped TiO2 Nanofibrous Membranes with Enhanced Photocatalytic Activity for Water Purification. Sci. Rep. 2017, 7, 1636. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Song, L.; Luo, L.; Zhang, Y.; Zhu, B.; Liu, J.; Chen, Z.; Zhang, L. Preparation of TiO2/C3N4 heterojunctions on carbon-fiber cloth as efficient filter-membrane-shaped photocatalyst for removing various pollutants from the flowing wastewater. J. Colloid Interface Sci. 2018, 532, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Geetha, D.; Nagarajan, E.R. Chapter 3—Impact and Issues of Organic Pollutants. In Management of Contaminants of Emerging Concern (CEC) in Environment; Singh, P., Hussain, C.M., Rajkhowa, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 93–126. [Google Scholar] [CrossRef]















| No. | Fundamental Reaction | Reaction Time (s) | |
|---|---|---|---|
| 1. | Generation of charge carriers | (1) | ~10−15 (very fast) |
| 2. | Trapping charge carriers | (2) | 10−8 (fast) |
| (3) | 10−10 (a) | ||
| (4) | 10−8 (b) | ||
| 3. | Charge carrier recombination | (5) | 10−7 (slow) |
| (6) | 10−8 (fast) | ||
| 4. | Interface charge carrier | (7) | 10−7 (slow) |
| (8) | ~10−6 (very slow) |
| 1D TiO2 Nanostructures | Synthesis Routes |
|---|---|
| TiO2 nanorods | sol-gel template, chemical vapor deposition (CVD), hydrothermal, wet-chemical |
| TiO2 nanotubes | template-assisted, hydrothermal, electrochemical deposition, sol-gel |
| TiO2 nanowires | hydrothermal, microwave-assisted, sol-gel electrophoretic deposition, solvothermal |
| TiO2 nanofibers | hydrothermal, electrospinning + sol-gel, hydrolysis |
| TiO2 nanobelts | solvothermal, hydrothermal, CVD |
| Doped Element(s) | Application | Method | Band Gap (eV) | Result | Reference | |
|---|---|---|---|---|---|---|
| Boron (B) | 4-NP degradation | Sol-gel | 2.95–2.98 | Under UV-light illumination, B-TiO2 generated 90% degradation of 4-NP (k = 0.0322 min−1), which was 11% higher than the pure TiO2 (k = 0.006 min−1). This improvement can be explained by the formation of Ti3+ due to B doping, which was responsible for enhancing the entrapment of photogenerated electron/hole as per the below reaction: | [67] | |
| (9) | ||||||
| Silver (Ag) | Photodegradation of nitrophenol | Sol-gel involving reducing agent | 3.1 | Optimized Ag-TiO2 anatase with the size of 6 nm, photodegradation reaction rate at 0.034 min−1, which was almost 1.9-times higher than the pure TiO2. | [68] | |
| Boron (B) | Phenol degradation | Sol-gel or Grinding- annealing | 2.85 | The B doping on TiO2 hindered the phase transformation from amorphous to anatase but can be active in the presence of visible light. However, excessive addition of B dopant resulted in B2O3 formation, which hampered the photoactivity. | [69] | |
| Ag-Sulfur (S) | Photocatalytic removal of 2-nitrophenol (2-NP) | Sol-gel | 2.39 | Under the solar experiment, the photodegradation and adsorption rate constant of Ag-S/TiO2 (Eg = 2.39 eV) obtained were 2.4- and 4.1-times larger than the bare TiO2 (Eg = 3.05 eV), respectively. The BET surface area and pore volume of Ag-S/TiO2 were found to be higher up to 60.6% and 30%, respectively. | [70] | |
| Transition metal (Iron (Fe), Vanadium (V), and Tungsten (W)) | Photocatalytic degradation of BTEX | Solvothermal | 3.14 (Fe-TiO2); 2.9 (W-TiO2 and V-TiO2) | The visible light catalytic activity of all doped TiO2 under is greater than that of pure TiO2. The V-TiO2 shows the highest photocatalytic activity followed by the W-TiO2 (54% conversion) and Fe-TiO2 (48% conversion) with the conversion of 69%. The UV-Vis diffuse reflectance spectra reveal that the V-TiO2 has the highest visible light absorption followed by the W-TiO2, Fe-TiO2, and undoped TiO2. The enhancement of TiO2 by transition metal doping is most likely due to the effect of the highest increase in visible light absorption as well as the smallest particle size among the V- TiO2 doped samples. | [71] | |
| Mangan (Mn) | Photooxidation of benzene, toluene, and xylene | Sol-gel | 3 | Under UV irradiation and in the appearance of oxygen, the degradation of benzene, toluene, and xylene by Mn-TiO2 can be achieved with the yields 92.6%, 82.9%, and 75%, respectively. The red-shift in the band gap energy upon Mn-doped TiO2 was observed, which shows the widening of UV-Vis absorption. | [72] | |
| Cerium (Ce) | Visible light degradation of toluene and o-xylene | Sonochemical synthesis | 2.89–3.07 | Photodegradation efficiencies of Ce-doped TiO2 (CeT) gained higher than undoped TiO2. The degradation efficiency by the CeT was consistent with the high desired surface area, pore size, and the particle size of the catalysts. Furthermore, the proper amount of Ce on TiO2 has less photoluminescent (PL) emission than undoped TiO2 and P25, indicating an increase in electron-hole separation and a reduction in photoexcited charge carrier recombination. | [73] | |
| Material(s) | Pollutant Target(s) | Result(s) | Mechanism | Reference | ||
|---|---|---|---|---|---|---|
| SSA (m2/g) | Band Gap (eV) | Rate Constant (min−1) | ||||
| CuO/WO3/TiO2 | Phenol; 4-chlorophenol (4-CP); 3-phenyl-1-propanol (3-P-1-P) | 41.1431 | 0.0621 (phenol); 0.017 (4-CP); 0.0108 (3-P-1-P) |  | [82] | |
| TiO2-WO3/ZSM-5 | xylene | 313.8 | 0.02826 |  | [83] | |
| TiO2/CdS | Phenol | 146.5 | 0.01266 |  | [84] | |
| TiO2(A)/TiO2(R)/ZnO | 4-CP | 3.0 | 0.0371 (UV); 0.0152 (Visible) |  | [85] | |
| TiO2(A)/TiO2(R)/SnO2 | 4-CP | 3.0 | 0.0234 (UV); 0.0102 (Visible) |  | [85] | |
| CeO2/TiO2 | Phenol | 76.9 | 2.88 | 0.0302 |  | [86] |
| Processing Step | Reaction |
|---|---|
| Fe3+-TiO2-Zeolite → Fe3+-TiO2-Zeolite (e− + h+) |
| Fe3+ + e− → Fe2+ Fe2+ + O2 → Fe3+ + O2• |
| O2• + H+ + e− → HO2• HO2• + Fe2+ + H+ ⇌ H2O2 + Fe3+ |
| Fe2+ + h+ → Fe3+ Fe2+ + H2O2 + H+ → Fe3+ + HO• Fe2+ + H2O + HO• → Fe3+ + H2O2 H2O2 + 2e− → 2HO• |
| Organic compounds + [Fe3+-TiO2-Zeolite (HO•, O2•, HO2•)] → Degraded product |
| Properties | Samples | |||
|---|---|---|---|---|
| NB | C-NB | PdO-NB | PdO-C-NB | |
| BET surface area (m2/g) | 30.82 | 34.29 | 42.72 | 48.67 |
| Pore size (nm) | 7.73 | 8.37 | 7.95 | 11.15 |
| Pore volume (m3/g) | 0.12 | 0.14 | 0.17 | 0.27 |
| Photocatalyst | Membrane and Module | Fabrication Method | Result(s) | Reference |
|---|---|---|---|---|
| TiO2/graphene oxide (GO) | Cellulose acetate/cellulose triacetate (CA/CTA) Flat-sheet | Immersion precipitation |
| [97] |
| TiO2 | Polyvinylidene fluoride (PVDF) Hollow-fiber | Dry-wet jet spinning |
| [98] |
| TiO2/carbon nanotubes (CNT) | Commercial PVDF Flat-sheet | Surface coating |
| [99] |
| TiO2 | Polyacryllonitrile (PAN) nanofibers Flat-sheet | Electrospray dispersion |
| [100] |
| Membrane | 3 h UV | 6 h UV | 6 h (Dark Controls) |
|---|---|---|---|
| Particles (108/mL) | |||
| Polyetersulfon (PES) | 0.75 | 2.4 | 0.2 |
| DK | 1.5 | 2 | 0.16 |
| BW30-400 (BW) | 0.23 | 2.2 | 0.39 |
| Cellulose acetate (CA) | 0.16 | 0.12 | 0.33 |
| NYLON | 0.27 | 0.53 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharma, H.N.C.; Jaafar, J.; Widiastuti, N.; Matsuyama, H.; Rajabsadeh, S.; Othman, M.H.D.; Rahman, M.A.; Jafri, N.N.M.; Suhaimin, N.S.; Nasir, A.M.; et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes 2022, 12, 345. https://doi.org/10.3390/membranes12030345
Dharma HNC, Jaafar J, Widiastuti N, Matsuyama H, Rajabsadeh S, Othman MHD, Rahman MA, Jafri NNM, Suhaimin NS, Nasir AM, et al. A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment. Membranes. 2022; 12(3):345. https://doi.org/10.3390/membranes12030345
Chicago/Turabian StyleDharma, Hadi Nugraha Cipta, Juhana Jaafar, Nurul Widiastuti, Hideto Matsuyama, Saied Rajabsadeh, Mohd Hafiz Dzarfan Othman, Mukhlis A Rahman, Nurul Natasha Mohammad Jafri, Nuor Sariyan Suhaimin, Atikah Mohd Nasir, and et al. 2022. "A Review of Titanium Dioxide (TiO2)-Based Photocatalyst for Oilfield-Produced Water Treatment" Membranes 12, no. 3: 345. https://doi.org/10.3390/membranes12030345