Photoreaction Pathways of Bacteriorhodopsin and Its D96N Mutant as Revealed by in Situ Photoirradiation Solid-State NMR
Abstract
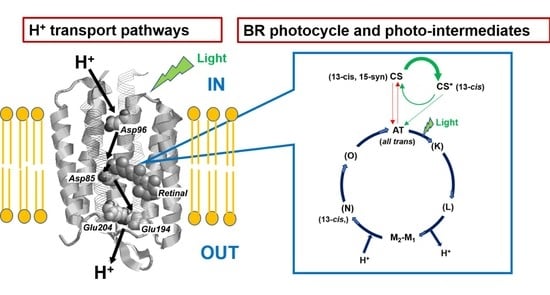
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. 13C and 15N Solid-State NMR Experiments
2.2.2. In Situ Photoirradiation Solid-State NMR Experiments
2.2.3. Observation of Photo-Intermediates and Photoreaction Pathways of BR
3. Results and Discussion
3.1. Photoreaction Pathway of [20-13C]retinal-BR under Illumination with Green Light at 20 °C
3.2. Photoreaction Pathways of [20-13C]retinal-BR under Illumination with Green Light at −20 °C
3.3. Photo-Reaction Cycle of [20-13C]retinal-BR
3.4. Photo-Reaction Pathways as Revealed by [20-13C, 14-13C]retinal-D96N-BR under Green Light Illumination from the DA to LA State at −30 °C
3.5. Photoreaction Pathways as Revealed by [14-13C, 20-13C]retinal-D96N-BR under Green Light Illumination from LA (D2) to DA (D3) States
3.6. Photoreaction Pathways as Revealed by [20-13C, 14-13C]retinal-D96N-BR under UV Light Illumination following Green Light Illumination
3.7. Photo-Reaction Pathways as Revealed by [ε-15N]Lys216-D96N-BR
3.8. Photo Reaction Pathways in the D96N-BR Mutant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanyi, J.K. Mechanism of Ion Transport across Membranes. J. Biol. Chem. 1997, 272, 31209–31212. [Google Scholar] [CrossRef] [Green Version]
- Facciotti, M.T.; Rouhani-Manshadi, S.; Glaeser, R.M. Energy transduction in transmembrane ion pumps. Trends Biochem. Sci. 2004, 29, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Ernst, O.P.; Lodowski, D.T.; Elstner, M.; Hegemann, P.; Brown, L.S.; Kandori, H. Microbial and Animal Rhodopsins: Structures, Functions, and Molecular Mechanisms. Chem. Rev. 2014, 114, 126–163. [Google Scholar] [CrossRef] [PubMed]
- Luecke, H.; Schobert, B.; Richter, H.-T.; Cartailler, J.-P.; Lanyi, J.K. Structure of bacteriorhodopsin at 1.55 Å resolution. J. Mol. Biol. 1999, 291, 899–911. [Google Scholar] [CrossRef] [Green Version]
- Luecke, H.; Schobert, B.; Cartailler, J.-P.; Richter, H.-T.; Rosengarth, A.; Needleman, R.; Lanyi, J.K. Coupling photoisomerization of retinal to directional transport in bacteriorhodopsin. J. Mol. Biol. 2000, 300, 1237–1255. [Google Scholar] [CrossRef] [Green Version]
- Lanyi, J.K. Proton transfers in the bacteriorhodopsin photocycle. Biochim. Biophys. Acta 2006, 1757, 1012–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nango, E.; Royant, A.; Kubo, M.; Nakane, T.; Wickstrand, C.; Kimura, T.; Tanaka, T.; Tono, K.; Song, C.; Tanaka, R.; et al. A three-dimensional movie of structural changes in bacteriorhodopsin. Science 2016, 354, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Nogly, P.; Weinert, T.; James, D.; Carbajo, S.; Ozerov, D.; Furrer, A.; Gashi, D.; Borin, V.; Skopintsev, P.; Jaeger, K.; et al. Retinal isomerization in bacteriorhodopsin captured by a femtosecond x-ray laser. Science 2018, 361, eaat0094. [Google Scholar] [CrossRef] [Green Version]
- Weinert, T.; Skopintsev, P.; James, D.; Dworkowski, F.; Panepucci, E.; Kekilli, D.; Furrer, A.; Brünle, S.; Mous, S.; Ozerov, D.; et al. Proton uptake mechanism in bacteriorhodopsin captured by serial synchrotron crystallography. Science 2019, 365, 61–65. [Google Scholar] [CrossRef]
- Lanyi, J.K. Molecular Mechanism of Ion Transport in Bacteriorhodopsin: Insights from Crystallographic, Spectroscopic, Kinetic, and Mutational Studies. J. Phys. Chem. B 2000, 104, 11441–11448. [Google Scholar] [CrossRef]
- Morgan, J.E.; Vakkasoglu, A.S.; Lanyi, J.K.; Lugtenburg, J.; Gennis, R.B.; Maeda, A. Structure Changes upon Deprotonation of the Proton Release Group in the Bacteriorhodopsin Photocycle. Biophys. J. 2012, 103, 444–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalisky, O.; Goldschmidt, C.; Ottolenghi, M. On the photocycle and light adaptation of dark-adapted bacteriorhodopsin. Biophys. J. 1977, 19, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Iwasa, T.; Tokunaga, F.; Yoshizawa, T. Photochemical reaction of 13-cis-bacteriorhodopsin studied by low temperature spec-trophotometry. Photochem. Photobiol. 1981, 33, 539–545. [Google Scholar] [CrossRef]
- Roepe, P.D.; Ahl, P.L.; Herzfeld, J.; Lugtenburg, J.; Rothschild, K. Tyrosine protonation changes in bacteriorhodopsin. A Fourier transform infrared study of BR548 and its primary photoproduct. J. Biol. Chem. 1988, 263, 5110–5117. [Google Scholar] [CrossRef]
- Logunov, I.; Humphrey, W.; Schulten, K.; Sheves, M. Molecular dynamics study of the 13-cis form (bR548) of bacteriorhodopsin and its photocycle. Biophys. J. 1995, 68, 1270–1282. [Google Scholar] [CrossRef] [Green Version]
- Richter, H.-T.; Needleman, R.; Lanyi, J.K. Perturbed interaction between residues 85 and 204 in Tyr-185→Phe and Asp-85→Glu bacteriorhodopsins. Biophys. J. 1996, 71, 3392–3398. [Google Scholar] [CrossRef] [Green Version]
- Dunach, M.; Marti, T.; Khorana, H.G.; Rothschild, K. Uv-visible spectroscopy of bacteriorhodopsin mutants: Substitution of Arg-82, Asp-85, Tyr-185, and Asp-212 results in abnormal light-dark adaptation. Proc. Natl. Acad. Sci. USA 1990, 87, 9873–9877. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Yang, D.; El-Sayed, M.A.; Lanyi, J.K. Retinal Isomer Composition in Some Bacteriorhodopsin Mutants under Light and Dark Adaptation Conditions. J. Phys. Chem. 1995, 99, 10052–10055. [Google Scholar] [CrossRef]
- Sonar, S.; Krebs, M.P.; Khorana, H.G.; Rothschild, K. Static and time-resolved absorption spectroscopy of the bacteriorhodopsin mutant Tyr-185.fwdarw. Phe: Evidence for an equilibrium between bR570 and an O-like species. Biochemistry 1993, 32, 2263–2271. [Google Scholar] [CrossRef]
- Rath, P.; Krebs, M.P.; He, Y.; Khorana, H.G.; Rothschild, K.J. Fourier transform Raman spectroscopy of the bacteriorhodopsin mutant Tyr-185→Phe: Formation of a stable O-like species during light adaptation and detection of its transient N-like pho-toproduct. Biochemistry 1993, 32, 2272–2281. [Google Scholar] [CrossRef]
- He, Y.; Krebs, M.P.; Fischer, W.B.; Khorana, H.G.; Rothschild, K.J. FTIR difference spectroscopy of the bacteriorhodopsin mutant Tyr-185→fwdarw. Phe: Detection of a stable O-like species and characterization of its photocycle at low temperature. Biochemistry 1993, 32, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Zimányi, L.; Varo, G.; Chang, M.; Ni, B.; Needleman, R.; Lanyi, J.K. Pathways of proton release in the bacteriorhodopsin photo-cycle. Biochemistry 1992, 31, 8535–8543. [Google Scholar] [CrossRef] [PubMed]
- Zimányi, L.; Kulcsár, Á.; Lanyi, J.K.; Sears, D.F.; Saltiel, J. Intermediate spectra and photocycle kinetics of the Asp96 → Asn mutant bacteriorhodopsin determined by singular value decomposition with self-modeling. Proc. Natl. Acad. Sci. USA 1999, 96, 4414–4419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikukawa, T.; Araiso, T.; Mukasa, K.; Shimozawa, T.; Kamo, N. The molecular motion of bacteriorhodopsin mutant D96N in the purple membrane. FEBS Lett. 1995, 377, 502–504. [Google Scholar] [CrossRef] [Green Version]
- Yao, B.; Zheng, Y.; Wang, Y.; Lei, M.; Chen, G.; Hampp, N. Kinetic spectra of light-adaptation dark-adaptation and M-intermediate of BR-D96N. Opt. Commun. 2003, 218, 125–130. [Google Scholar] [CrossRef]
- Lakshmi, K.V.; Farrar, M.R.; Raap, J.; Lugtenburg, J.; Griffin, R.G.; Herzfeld, J. Solid State 13C and 15N NMR Investigations of the N Intermediate of Bacteriorhodopsin. Biochemistry 1994, 33, 8853–8857. [Google Scholar] [CrossRef]
- Hu, J.G.; Sun, B.Q.; Petkova, A.T.; Griffin, R.G.; Herzfeld, J. The predischarge chromophore in bacteriorhodopsin: A 15N sol-id-state NMR study of the L photointermediate. Biochemistry 1997, 36, 9316–9322. [Google Scholar] [CrossRef]
- Harbison, G.S.; Smith, S.O.; Pardoen, J.A.; Winkel, C.; Lugtenburg, J.; Herzfeld, J.; Mathies, R.; Griffin, R.G. Dark-adapted bac-teriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc. Natl. Acad. Sci. USA 1984, 81, 1706–1709. [Google Scholar] [CrossRef] [Green Version]
- Harbison, G.S.; Smith, S.O.; Pardoen, J.A.; Mulder, P.P.; Lugtenburg, J.; Herzfeld, J.; Mathies, R.; Griffin, R.G. Solid-state 13C NMR studies of retinal in bacteriorhodopsin. Biochemistry 1984, 23, 2662–2667. [Google Scholar] [CrossRef]
- Smith, S.O.; de Groot, H.; Gebhard, R.; Courtin, J.M.L.; Lugtenburg, J.; Herzfeld, J.; Griffin, R.G. Structure and protein environment of the retinal chromophore in light- and dark-adapted bacteriorhodopsin studied by solid-state NMR. Biochemistry 1989, 28, 8897–8904. [Google Scholar] [CrossRef]
- Hu, J.G.; Sun, B.Q.; Bizounok, M.; Hatcher, M.E.; Lansing, J.C.; Raap, J.; Verdegem, P.J.E.; Lugtenburg, J.; Griffin, R.G.; Herzfeld, J. Early and Late M Intermediates in the Bacteriorhodopsin Photocycle: A Solid-State NMR Study. Biochemistry 1998, 37, 8088–8096. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, V.S.; Mak-Jurkauskas, M.L.; Belenky, M.; Herzfeld, J.; Griffin, R.G. Functional and shunt states of bacteriorhodopsin re-solved by 250 GHz dynamic nuclear polarization-enhanced solid-state NMR. Proc. Natl. Acad. Sci. USA 2009, 106, 9244–9249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, I.; Kihara, N.; Ohmine, M.; Nishimura, K.; Tuzi, S.; Saitô, H.; Naito, A. Solid-state NMR studies of two backbone conformations at Tyr185 as a function of retinal configurations in the dark, light, and pressure adapted bacteriorhodopsins. J. Am. Chem. Soc. 2007, 129, 1016–1017. [Google Scholar] [CrossRef]
- Petkova, A.T.; Hatanaka, M.; Jaroniec, C.P.; Hu, J.G.; Belenky, M.; Verhoeven, M.; Lugtenburg, J.; Griffin, R.G.; Herzfeld, J. Tryp-tophan interaction in bacteriorhodopsin: A heteronuclear solid-state NMR study. Biochemistry 2002, 41, 2429–2437. [Google Scholar] [CrossRef]
- Mak-Jurkauskas, M.L.; Bajaj, V.S.; Hornstein, M.K.; Belenky, M.; Griffin, R.G.; Herzfeld, J. Energy transformations early in the bacteriorhodopsin photocycle revealed by DNP-enhanced solid-state NMR. Proc. Natl. Acad. Sci. USA 2008, 105, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Becker-Baldus, J.; Bamann, C.; Saxena, K.; Gustmann, H.; Brown, L.J.; Brown, R.C.D.; Reiter, C.; Bamberg, E.; Wachtveitl, J.; Schwalbe, H.; et al. Enlightening the photoactive site of channelrhodopsin-2 by DNP-enhanced solid-state NMR spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, 9896–9901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomonaga, Y.; Hidaka, T.; Kawamura, I.; Nishio, T.; Ohsawa, K.; Okitsu, T.; Wada, A.; Sudo, Y.; Kamo, N.; Ramamoorthy, A.; et al. An Active Photoreceptor Intermediate Revealed by In Situ Photoirradiated Solid-State NMR Spectroscopy. Biophys. J. 2011, 101, L50–L52. [Google Scholar] [CrossRef] [Green Version]
- Yomoda, H.; Makino, Y.; Tomonaga, Y.; Hidaka, T.; Kawamura, I.; Okitsu, T.; Wada, A.; Sudo, Y.; Naito, A. Color-Discriminating Retinal Configurations of Sensory Rhodopsin I by Photo-Irradiation Solid-State NMR Spectroscopy. Angew. Chem. Int. Ed. 2014, 53, 6960–6964. [Google Scholar] [CrossRef]
- Makino, Y.; Kawamura, I.; Okitsu, T.; Wada, A.; Kamo, N.; Sudo, Y.; Ueda, K.; Naito, A. Retinal Configuration of ppR Intermediates Revealed by Photoirradiation Solid-State NMR and DFT. Biophys. J. 2018, 115, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Naito, A.; Kawamura, I. Photo-Activated Structural Changes in Photoreceptor Membrane Proteins as Revealed by In Situ Pho-Toirradiation Solid-State NMR; Advances in Biological Solid-State NMR: Proteins and Membrane Active peptides; Separovic, F., Naito, A., Eds.; Royal Society of Chemistry: London, UK, 2014; pp. 387–404. [Google Scholar]
- Naito, A.; Kawamura, I.; Javkhlantugs, N. Recent Solid-State NMR Studies of Membrane-Bound Peptides and Proteins. Annu. Rep. NMR Spectrosc. 2015, 86, 333–411. [Google Scholar] [CrossRef]
- Naito, A.; Makino, Y.; Kawamura, I. In Situ Photo Irradiation Solid-State NMR Spectroscopy Applied to Retinal-Binding Membrane Proteins. In Modern Magnetic Resonance, 2nd ed.; Springer: Berlin, Germany, 2018; pp. 537–557. [Google Scholar]
- Naito, A.; Makino, Y.; Tasei, Y.; Kawamura, I. Photoirradiation and Microwave Irradiation NMR Spectroscopy. Exp. Approaches NMR Spectrosc. 2018, 5, 135–170. [Google Scholar] [CrossRef]
- Oshima, K.; Shigeta, A.; Makino, Y.; Kawamura, I.; Okitsu, T.; Wada, A.; Tuzi, S.; Iwasa, T.; Naito, A. Characterization of pho-to-intermediates in the photo-reaction pathways of a bacteriorhodopsin Y185F mutant using in situ photo-irradiation solid-state NMR spectroscopy. Photochem. Photobiol. Sci. 2015, 14, 1694–1702. [Google Scholar] [CrossRef]
- Naito, A.; Makino, Y.; Shigeta, A.; Kawamura, I. Photoreaction pathways and photointermediates of retinal-binding photore-ceptor proteins as revealed by in situ photoirradiation solid-state NMR spectroscopy. Biophys. Rev. 2019, 11, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, D.; Stoeckenious, W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974, 31, 667–678. [Google Scholar] [PubMed]
- Kawamura, I.; Tanabe, J.; Ohmine, M.; Yamaguchi, S.; Tuzi, S.; Naito, A. Participation of the BC Loop in the Correct Folding of Bacteriorhodopsin as Revealed by Solid-state NMR. Photochem. Photobiol. 2009, 85, 624–630. [Google Scholar] [CrossRef]
- Bennett, A.E.; Rienstra, C.M.; Auger, M.; Lakshmi, K.V.; Griffin, R.G. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 1995, 103, 6951–6958. [Google Scholar] [CrossRef]
- Druckmann, S.; Friedman, N.; Lanyi, J.K.; Needleman, R.; Ottolenghi, M.; Sheves, M. The back photoreaction of the M intermediate in the photocycle of bacteriorhodopsin: Mechanism and evidence for two M species. Photochem. Photobiol. 1992, 56, 1041–1047. [Google Scholar] [CrossRef]








| Protein | State | Temp. | 20-13C | 14-13C | [ε-15N]Lys216 | Configuration |
|---|---|---|---|---|---|---|
| WT-BR | AT(BR568) | 20 °C | 13.1 | all-trans | ||
| −20 °C | 13.3 | all-trans | ||||
| CS(BR548) | 20 °C | 22.1 | 13-cis | |||
| −20 °C | 22.3 | 13-cis | ||||
| CS* | −20 °C | 19.6 | 13-cis | |||
| N | −20 °C | 19.8 | 13-cis | |||
| D96N | AT(BR568) | −30 °C | 13.1 | 121.8 | all-trans | |
| −60 °C | 148 | all-trans | ||||
| CS(BR548) | −30 °C | 22.6 | 110.1 | 13-cis, 15-syn | ||
| −60 °C | 153 | 13-cis, 15-syn | ||||
| CS* | −30 °C | 20.0 | 115.8 | 13-cis | ||
| M | −30 °C | 20.0 | 124.1 | 13-cis, 15-anti | ||
| −60 °C | 300 | 13-cis, 15-anti | ||||
| L | −30 °C | 18.6 | 115.8 | 13-cis, |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigeta, A.; Otani, Y.; Miyasa, R.; Makino, Y.; Kawamura, I.; Okitsu, T.; Wada, A.; Naito, A. Photoreaction Pathways of Bacteriorhodopsin and Its D96N Mutant as Revealed by in Situ Photoirradiation Solid-State NMR. Membranes 2022, 12, 279. https://doi.org/10.3390/membranes12030279
Shigeta A, Otani Y, Miyasa R, Makino Y, Kawamura I, Okitsu T, Wada A, Naito A. Photoreaction Pathways of Bacteriorhodopsin and Its D96N Mutant as Revealed by in Situ Photoirradiation Solid-State NMR. Membranes. 2022; 12(3):279. https://doi.org/10.3390/membranes12030279
Chicago/Turabian StyleShigeta, Arisu, Yuto Otani, Ryota Miyasa, Yoshiteru Makino, Izuru Kawamura, Takashi Okitsu, Akimori Wada, and Akira Naito. 2022. "Photoreaction Pathways of Bacteriorhodopsin and Its D96N Mutant as Revealed by in Situ Photoirradiation Solid-State NMR" Membranes 12, no. 3: 279. https://doi.org/10.3390/membranes12030279