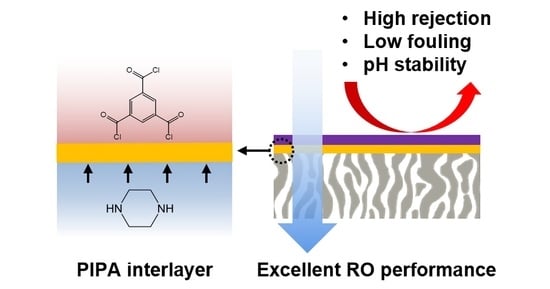
Highly Selective and pH-Stable Reverse Osmosis Membranes Prepared via Layered Interfacial Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Membrane Performance and pH Stability
3. Results and Discussion
3.1. Membrane Performance
3.2. Membrane Structure and Properties
3.3. Membrane pH Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, J.; Yang, D.R.; Hong, S. Towards a low-energy seawater reverse osmosis desalination plant: A review and theoretical analysis for future directions. J. Membr. Sci. 2020, 595, 117607. [Google Scholar] [CrossRef]
- Giwa, A.; Akther, N.; Dufour, V.; Hasan, S.W. A critical review on recent polymeric and nano-enhanced membranes for reverse osmosis. RSC Adv. 2016, 6, 8134–8163. [Google Scholar] [CrossRef]
- Li, D.; Yan, Y.S.; Wang, H.T. Recent advances in polymer and polymer composite membranes for reverse and forward osmosis processes. Prog. Polym. Sci. 2016, 61, 104–155. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Xu, J.; Yan, H.; Zhang, Y.; Pan, G.Y.; Liu, Y.Q. The morphology of fully-aromatic polyamide separation layer and its relationship with separation performance of TFC membranes. J. Membr. Sci. 2017, 541, 174–188. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2016, 81, 209–237. [Google Scholar] [CrossRef]
- Shang, C.; Pranantyo, D.; Zhang, S. Understanding the roughness-fouling relationship in reverse osmosis: Mechanism and implications. Environ. Sci. Technol. 2020, 54, 5288–5296. [Google Scholar] [CrossRef]
- Gu, J.E.; Lee, S.; Stafford, C.M.; Lee, J.S.; Choi, W.; Kim, B.Y.; Baek, K.Y.; Chan, E.P.; Chung, J.Y.; Bang, J.; et al. Molecular layer-by-layer assembled thin-film composite membranes for water desalination. Adv. Mater. 2013, 25, 4778–4782. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Choi, W.; Gu, J.E.; Park, S.H.; Kim, S.; Bang, J.; Baek, K.Y.; Park, B.; Lee, J.S.; Chan, E.P.; Lee, J.H. Tailor-made polyamide membranes for water desalination. ACS Nano 2015, 9, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Choi, W.; Nam, S.E.; Hong, S.; Lee, J.S.; Lee, J.H. Fabrication of polyamide thin film composite reverse osmosis membranes via support-free interfacial polymerization. J. Membr. Sci. 2017, 526, 52–59. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Wang, J.Q.; Fang, L.F.; Lin, H.B.; Liu, F.; Tang, C.Y.Y. Electrosprayed polyamide nanofiltration membrane with intercalated structure for controllable structure manipulation and enhanced separation performance. J. Membr. Sci. 2020, 602, 117971. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Steffes, J.; Huey, B.D.; McCutcheon, J.R. 3D printed polyamide membranes for desalination. Science 2018, 361, 682–685. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Ahn, W.G.; Choi, W.; Park, S.H.; Lee, J.S.; Jung, H.W.; Lee, J.H. A facile and scalable fabrication method for thin film composite reverse osmosis membranes: Dual-layer slot coating. J. Mater. Chem. A 2017, 5, 6648–6655. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.H. Fabrication of high-performance reverse osmosis membranes via dual-layer slot coating with tailoring interfacial adhesion. J. Membr. Sci. 2020, 614, 118449. [Google Scholar] [CrossRef]
- Jiang, Z.; Karan, S.; Livingston, A.G. Water transport through ultrathin polyamide nanofilms used for reverse osmosis. Adv. Mater. 2018, 30, 1705973. [Google Scholar] [CrossRef]
- Choi, W.; Jeon, S.; Kwon, S.J.; Park, H.; Park, Y.I.; Nam, S.E.; Lee, P.S.; Lee, J.S.; Choi, J.; Hong, S.; et al. Thin film composite reverse osmosis membranes prepared via layered interfacial polymerization. J. Membr. Sci. 2017, 527, 121–128. [Google Scholar] [CrossRef]
- Choi, W.; Lee, C.; Lee, D.; Won, Y.J.; Lee, G.W.; Shin, M.G.; Chun, B.; Kim, T.S.; Park, H.D.; Jung, H.W.; et al. Sharkskin- mimetic desalination membranes with ultralow biofouling. J. Mater. Chem. A 2018, 6, 23034–23045. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. A review on semi-aromatic polyamide TFC membranes prepared by interfacial polymerization: Potential for water treatment and desalination. Sep. Purif. Technol. 2017, 181, 159–182. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhao, Y.Y.; Wang, X.M.; Wen, X.H.; Huang, X.; Xie, Y.F.F. Effect of varying piperazine concentration and post-modification on prepared nanofiltration membranes in selectively rejecting organic micropollutants and salts. J. Membr. Sci. 2019, 582, 274–283. [Google Scholar] [CrossRef]
- Shin, M.G.; Seo, J.Y.; Park, H.; Park, Y.I.; Ji, S.; Lee, S.S.; Lee, J.H. Positively charged membranes with fine-tuned nanopores for ultrafast and high-precision cation separation. J. Mater. Chem. A 2021, 9, 24355–24364. [Google Scholar] [CrossRef]
- Dalwani, M.; Benes, N.E.; Bargeman, G.; Stamatialis, D.; Wessling, M. Effect of pH on the performance of polyamide/polyacrylonitrile based thin film composite membranes. J. Membr. Sci. 2011, 372, 228–238. [Google Scholar] [CrossRef]
- Huang, S.H.; Hsu, C.J.; Liaw, D.J.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Effect of chemical structures of amines on physicochemical properties of active layers and dehydration of isopropanol through interfacially polymerized thin-film composite membranes. J. Membr. Sci. 2008, 307, 73–81. [Google Scholar] [CrossRef]
- Klaysom, C.; Hermans, S.; Gahlaut, A.; Van Craenenbroeck, S.; Vankelecom, I.F.J. Polyamide/Polyacrylonitrile (PA/PAN) thin film composite osmosis membranes: Film optimization, characterization and performance evaluation. J. Membr. Sci. 2013, 445, 25–33. [Google Scholar] [CrossRef]
- Chai, G.Y.; Krantz, W.B. Formation and characterization of polyamide membranes via interfacial polymerization. J. Membr. Sci. 1994, 93, 175–192. [Google Scholar] [CrossRef]
- An, Q.F.; Li, F.; Ji, Y.L.; Chen, H.L. Influence of polyvinyl alcohol on the surface morphology, separation and anti-fouling performance of the composite polyamide nanofiltration membranes. J. Membr. Sci. 2011, 367, 158–165. [Google Scholar] [CrossRef]
- Wei, J.; Liu, X.; Qiu, C.Q.; Wang, R.; Tang, C.Y.Y. Influence of monomer concentrations on the performance of polyamide-based thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 381, 110–117. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Tang, C.Y.Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Membr. Sci. 2019, 590, 117297. [Google Scholar] [CrossRef]
- Klaysom, C.; Cath, T.Y.; Depuydt, T.; Vankelecom, I.F. Forward and pressure retarded osmosis: Potential solutions for global challenges in energy and water supply. Chem. Soc. Rev. 2013, 42, 6959–6989. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Hoek, E.M.V. Impacts of support membrane structure and chemistry on polyamide-polysulfone interfacial composite membranes. J. Membr. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Xu, J.; Gao, C. Thin film composite forward osmosis membrane with single-walled carbon nanotubes interlayer for alleviating internal concentration polarization. Polymers 2020, 12, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Li, C.; Li, S.; Su, B.; Hu, M.Z.; Gao, X.; Gao, C. Graphene quantum dots (GQDs)-polyethyleneimine as interlayer for the fabrication of high performance organic solvent nanofiltration (OSN) membranes. Chem. Eng. J. 2020, 380, 122462. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, M.S.; Choi, W.; Lee, J.H. Biocidal surfactant-assisted fabrication of thin film composite membranes with excellent and durable anti-biofouling performance. Chem. Eng. J. 2022, 431, 134114. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, J.H. Rationally designed in-situ fabrication of thin film nanocomposite membranes with enhanced desalination and anti-biofouling performance. J. Membr. Sci. 2020, 615, 118542. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Z.; Xu, J.; Wang, J.; Wang, S. Influence of hexamethyl phosphoramide on polyamide composite reverse osmosis membrane performance. Sep. Purif. Technol. 2010, 75, 145–155. [Google Scholar] [CrossRef]
- Jeon, S.; Park, C.H.; Shin, S.S.; Lee, J.-H. Fabrication and structural tailoring of reverse osmosis membranes using β-cyclodextrin-cored star polymers. J. Membr. Sci. 2020, 611, 118415. [Google Scholar] [CrossRef]
- Lee, H.D.; Kim, H.W.; Cho, Y.H.; Park, H.B. Experimental evidence of rapid water transport through carbon nanotubes embedded in polymeric desalination membranes. Small 2014, 10, 2653–2660. [Google Scholar] [CrossRef]
- Park, S.H.; Kwon, S.J.; Shin, M.G.; Park, M.S.; Lee, J.S.; Park, C.H.; Park, H.; Lee, J.H. Polyethylene-supported high performance reverse osmosis membranes with enhanced mechanical and chemical durability. Desalination 2018, 436, 28–38. [Google Scholar] [CrossRef]
- Freger, V. Nanoscale heterogeneity of polyamide membranes formed by interfacial polymerization. Langmuir 2003, 19, 4791–4797. [Google Scholar] [CrossRef]
- Kim, I.C.; Jegal, J.; Lee, K.H. Effect of aqueous and organic solutions on the performance of polyamide thin-film-composite nanofiltration membranes. J. Polym. Sci. B Polym. Phys. 2002, 40, 2151–2163. [Google Scholar] [CrossRef]
- Park, C.H.; Jeon, S.; Park, S.H.; Shin, M.G.; Park, M.S.; Lee, S.Y.; Lee, J.H. Cellulose nanocrystal-assembled reverse osmosis membranes with high rejection performance and excellent antifouling. J. Mater. Chem. A 2019, 7, 3992–4001. [Google Scholar] [CrossRef]
- Jeon, S.; Park, C.H.; Park, S.H.; Shin, M.G.; Kim, H.J.; Baek, K.Y.; Chan, E.P.; Bang, J.; Lee, J.H. Star polymer-assembled thin film composite membranes with high separation performance and low fouling. J. Membr. Sci. 2018, 555, 369–378. [Google Scholar] [CrossRef]
- Li, Q.L.; Xu, Z.H.; Pinnau, I. Fouling of reverse osmosis membranes by biopolymers in wastewater secondary effluent: Role of membrane surface properties and initial permeate flux. J. Membr. Sci. 2007, 290, 173–181. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Wang, X.M.; Yang, H.W.; Xie, Y.F.F. Effects of organic fouling and cleaning on the retention of pharmaceutically active compounds by ceramic nanofiltration membranes. J. Membr. Sci. 2018, 563, 734–742. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, J.; Feng, S.; Chen, X.; Luo, J.; Wan, Y. Tuning pore size and surface charge of poly (piperazinamide) nanofiltration membrane by enhanced chemical cleaning treatment. J. Membr. Sci. 2021, 120054. [Google Scholar] [CrossRef]
- Gu, J.E.; Lee, J.S.; Park, S.H.; Kim, I.T.; Chan, E.P.; Kwon, Y.N.; Lee, J.H. Tailoring interlayer structure of molecular layer-by-layer assembled polyamide membranes for high separation performance. Appl. Surf. Sci. 2015, 356, 659–667. [Google Scholar] [CrossRef]
- Wang, N.X.; Zhang, G.J.; Ji, S.L.; Qin, Z.P.; Liu, Z.Z. The salt-, pH- and oxidant-responsive pervaporation behaviors of weak polyelectrolyte multilayer membranes. J. Membr. Sci. 2010, 354, 14–22. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Sukhishvili, S.A. Ionization and pH stability of multilayers formed by self-assembly of weak polyelectrolytes. Langmuir 2003, 19, 1235–1243. [Google Scholar] [CrossRef]
- Tong, W.J.; Gao, C.Y.; Mohwald, H. Stable weak polyelectrolyte microcapsules with pH-responsive permeability. Macromolecules 2006, 39, 335–340. [Google Scholar] [CrossRef]
- Lavalle, P.; Voegel, J.C.; Vautier, D.; Senger, B.; Schaaf, P.; Ball, V. Dynamic aspects of films prepared by a sequential deposition of species: Perspectives for smart and responsive materials. Adv. Mater. 2011, 23, 1191–1221. [Google Scholar] [CrossRef] [PubMed]










| Membrane | A (LMH bar−1) | RNaCl (%) | Reference | |
|---|---|---|---|---|
| Commercial | SW30LE | 1.11 ± 0.11 | 98.8 ± 0.1 | This study |
| SW30HR | 1.03 ± 0.41 | 97.6 ± 1.1 | ||
| SWC4+ | 1.44 ± 0.23 | 99.6 ± 0.5 | ||
| BW30 | 2.93 ± 0.21 | 98.4 ± 0.3 | ||
| BW30LE | 3.76 ± 0.63 | 97.3 ± 0.5 | ||
| Lab-made | 1.56 ± 0.24 | 98.7 ± 0.5 | [19] | |
| 1.60 ± 0.10 | 99.6 ± 0.3 | [35] | ||
| 1.85 ± 0.08 | 99.3 ± 0.2 | [36] | ||
| 3.74 | 98.3 | [37] | ||
| 1.43 ± 0.08 | 99.0 ± 0.3 | [38] | ||
| 2.74 | 98.2 | [39] | ||
| 1.96 ± 0.14 | 99.5 ± 0.1 | [40] | ||
| 1.59 ± 0.09 | 99.8 ± 0.2 | This study (pLIP) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, M.-G.; Choi, W.; Lee, J.-H. Highly Selective and pH-Stable Reverse Osmosis Membranes Prepared via Layered Interfacial Polymerization. Membranes 2022, 12, 156. https://doi.org/10.3390/membranes12020156
Shin M-G, Choi W, Lee J-H. Highly Selective and pH-Stable Reverse Osmosis Membranes Prepared via Layered Interfacial Polymerization. Membranes. 2022; 12(2):156. https://doi.org/10.3390/membranes12020156
Chicago/Turabian StyleShin, Min-Gyu, Wansuk Choi, and Jung-Hyun Lee. 2022. "Highly Selective and pH-Stable Reverse Osmosis Membranes Prepared via Layered Interfacial Polymerization" Membranes 12, no. 2: 156. https://doi.org/10.3390/membranes12020156