Effect of Phase Heterogeneity on the Properties of Poly(vinyl alcohol)-Based Composite Pervaporation Membranes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.1.1. Film Preparation
2.1.2. Preparation of Film Membranes
2.1.3. Preparation of Bilayer Composite Membranes
2.1.4. Preparation of MMM-Type Membranes
2.2. Methods
2.2.1. X-ray Diffraction (XRD) Analysis
2.2.2. Atomic Force Microscopy (AFM)
2.2.3. Differential Scanning Calorimetry (DSC) and Thermal Gravimetric Analysis
2.2.4. IR Spectroscopy
2.3. Pervaporation Experiments
2.3.1. Vacuum Pervaporation
- (i)
- A thermostatically controlled steel membrane module (a non-continuous water-thermostated PV cell equipped with the mixing device that provided the uniform density of the feed mixture). The working surface area of the membrane was 6.07 × 10−4 m2;
- (ii)
- A vacuum pump, with which the permeate was removed by vacuum degassing followed by condensation in a liquid-nitrogen-cooled trap;
- (iii)
- A receiver for collecting the permeate cooled with liquid nitrogen ensured the complete condensation of the vapors that passed through the membrane;
- (iv)
- A pressure gauge that allowed for controlling the pressure under the membrane (3–5 mm Hg).
2.3.2. Analysis of Compositions of Liquid Mixtures
Refractometry
Chromatography
3. Results and Discussion
3.1. The Structures of Films Based on PVA, PAA, and PVA-PAA Blends
3.2. Pervaporation Properties of PVA, PAA, and Membranes Prepared from Their Blends
3.3. Structure and Pervaporation Properties of PVA, pDMAEMA, and the Membranes Prepared from Their Blends
3.3.1. Structural Features
3.3.2. Transport Properties of the PVA–pDMAEMA Composite Membranes
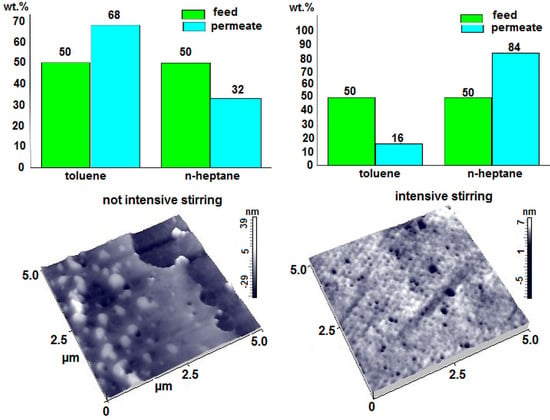
3.4. Formation of More Permeable Composite Membranes with Diffusion Layers Based on PVA-PAA
3.4.1. Composite Membranes with the PVA-PAA Diffusion Layers on a Porous Support
3.4.2. Composite Membranes of the MMM Type
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVA | poly(vinyl alcohol) |
| PAA | poly(acrylic acid) |
| pDMAEMA | poly(N,Ndimethylaminoethyl methacrylate) |
| XRD | X-ray diffraction |
| AFM | atomic force microscopy |
| DSC | differential scanning calorimetry |
| ATR IR | attenuated total reflection infrared spectra |
| PV | pervaporation |
| DS | the degree of saponification |
References
- Yihun, F.A.; Ifuku, S.; Saimoto, H.; Yihun, D.A. Thermo-mechanically improved polyvinyl alcohol composite films using maleated chitin nanofibers as nano-reinforcement. Cellulose 2021, 28, 2965–2980. [Google Scholar] [CrossRef]
- Ren, G.; Xu, X.; Liu, Q.; Cheng, J.; Yuan, X.; Wu, L.; Wan, Y. Electrospun poly(vinyl alcohol)/glucose oxidase biocomposite membranes for biosensor applications. React. Funct. Polym. 2006, 66, 1559–1564. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef] [PubMed]
- Yeum, J.H.; Ji, B.C.; Noh, S.K.; Jeon, H.Y.; Kwak, J.W.; Lyoo, W.S. Effect of syndiotacticity on the morphology of water-soluble low molecular weight poly(vinyl alcohol) by solution copolymerization of vinyl pivalate/vinyl acetate in tetrahydrofuran and saponification. Polymer 2004, 45, 4037–4043. [Google Scholar] [CrossRef]
- Nagarkar, R.; Patel, J. Polyvinyl alcohol: A comprehensive study. ASPS 2019, 3, 34–44. [Google Scholar]
- Guirguis, O.W.; Moselhey, M.T.H. Thermal and structural studies of poly(vinyl alcohol) and hydroxypropyl cellulose blends. Nat. Sci. 2012, 4, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Zadeh, S.N.; Rajabnezhad, S.; Zandkarimi, M.; Dahmardeh, S.; Mir, L. Mucoadhesive microspheres of chitosan and polyvinyl alcohol as a carrier for intranasal delivery of insulin: In vitro and in vivo studies. MOJ Bioequiv. Availab. 2017, 3, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Samzadeh-Kermani, A.; Mirzaee, M.; Ghaffari-Moghaddam, M. Polyvinyl alcohol/polyaniline/ZnOnanocomposite: Synthesis, characterization and bactericidal property. Adv. Biol. Chem. 2016, 6, 63314. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Huang, R.Y.M.; Feng, X. Preparation and properties of trimesoyl chloride crosslinked poly(vinyl alcohol) membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2006, 286, 245–254. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.-S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Hasimi, A.; Stavropoulou, A.; Papadokostaki, K.G.; Sanopoulou, M. Transport of water in polyvinyl alcohol films: Effect of thermal treatment and chemical crosslinking. Eur. Polym. J. 2008, 44, 4098–4107. [Google Scholar] [CrossRef]
- Mansur, H.S.; Mansur, A.A.P. Small angle X-ray scattering, FTIR and SEM characterization of nanostructured PVA/TEOS hybrids by chemical crosslinking. Mater. Res. Soc. Symp. Proc. 2005, 873, 20–25. [Google Scholar] [CrossRef]
- Liu, L.; Kentish, S.E. Pervaporation performance of crosslinked PVA membranes in the vicinity of the glass transition temperature. J. Membr. Sci. 2018, 553, 63–69. [Google Scholar] [CrossRef]
- Moritani, T.; Kajitani, K. Functional modification of poly(vinyl alcohol) by copolymerization: 1. Modification with carboxylic monomers. Polymer 1997, 38, 2933–2945. [Google Scholar] [CrossRef]
- Maruhashi, M. Polyvinyl Alcohol; Finch, C.A., Ed.; Wiley: New York, NY, USA, 1992; Volume II, Chapter 7. [Google Scholar]
- Ruckenstein, E.; Liang, L. Poly(acry1ic acid)—Poly(viny1 alcohol) semi- and interpenetrating polymer network pervaporation membranes. J. Appl. Polym. Sci. 1996, 62, 973–987. [Google Scholar] [CrossRef]
- Kumeta, K.; Nagashima, I.; Matsui, S.; Mizoguchi, K. Crosslinking Reaction of Poly(vinyl alcohol) with Poly(acrylic acid) (PAA) by Heat Treatment: Effect of Neutralization of PAA. J. Appl. Polym. Sci. 2003, 90, 2420–2427. [Google Scholar] [CrossRef]
- Raeisi, Z.; Hosseinzadeh, L.; Moheb, A.; Sadeghi, M. PVA-based Pervaporation Membranes for Separation of Water-Alcohol Solutions: A Review. Anal. Bioanal. Chem. Res. 2021, 8, 219–243. [Google Scholar] [CrossRef]
- Burshe, M.C.; Sawant, S.B.; Joshi, J.B.; Pangarkar, V.G. Dehydration of ethylene glycol by pervaporation using hydrophilic IPNs of PVA, PAA and PAAM membranes. Sep. Purif. Technol. 1998, 13, 47–56. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Chaudhari, S.; Moon, M.J.; Shon, M.Y.; Park, A.; Kim, Y.M. Pervaporation Dehydration of Acetic Acid Aqueous Solution using PVA/PAAMembrane with Na-Y Zeolite. Korean Chem. Eng. Res. 2017, 55, 778–784. [Google Scholar] [CrossRef]
- Pandey, L.K.; Saxena, C.; Dubey, V. Modification of poly(vinyl alcohol) membranes for pervaporative separation of benzene/cyclohexane mixtures. J. Membr. Sci. 2003, 227, 173–182. [Google Scholar] [CrossRef]
- Sagar, R.; Singha, N.R. Polymeric Nanocomposite Membranes for Next Generation Pervaporation Process: Strategies, Challenges and Future Prospects. Membranes 2017, 7, 53. [Google Scholar] [CrossRef]
- Sarkhel, D.; Roy, D.; Bandyopadhway, M.; Madhusree, B.; Bhattacharya, P. Studies on separation characteristics and pseudo-equilibrium relationship in pervaporation of benzene-cyclohexane mixtures through composite PVA membranes on PAN supports. Sep. Purif. Technol. 2003, 30, 89–96. [Google Scholar] [CrossRef]
- Yamasaki, A.; Shinbo, T.; Mizoguchi, K. Pervaporation of benzene/cyclohexane and benzene/n-hexane mixtures through PVA membranes. J. Appl. Polym. Sci. 1997, 64, 1061–1065. [Google Scholar] [CrossRef]
- Bryant, D.L.; Noble, R.D. Facilitated transport separation of benzene and cyclohexane with poly(vinyl alcohol)-AgNO3 membranes. J. Membr. Sci. 1997, 127, 161–170. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Z.; Hu, C.; Wang, Y.; Lu, L.; Wu, H. Pervaporation of benzene/cyclohexane mixtures through poly(vinyl alcohol) membranes with and without β-cyclodextrin. Desalination 2006, 193, 182–192. [Google Scholar] [CrossRef]
- Villaluenga, J.P.G.; Tabe-Mohammadi, A. A review on the separation of benzene/cyclohexane mixtures by pervaporation processes. J. Membr. Sci. 2000, 169, 159–174. [Google Scholar] [CrossRef]
- Liu, H.-X.; Wang, N.; Zhao, C.; Ji, S.; Li, J.-R. Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures. A review. Chin. J. Chem. Eng. 2018, 26, 1–16. [Google Scholar] [CrossRef]
- Bruschke, H.E.A. State of the art of the pervaporation process in chemical industry. In Membrane Technology; Peinemann, K.-V., Nunes, S., Eds.; Wiley VCH: Weinheim, Germany, 2001; Chapter II-3; pp. 127–172. [Google Scholar]
- Mandal, M.K.; Bhattacharya, P.K. Poly(vinyl acetal) membrane for pervaporation of benzene-isooctane solution. Sep. Purif. Technol. 2008, 61, 332–340. [Google Scholar] [CrossRef]
- Peng, F.; Pan, F.; Sun, H.; Lu, L.; Jiang, Z. Novel nanocompositepervaporation membranes composed of poly(vinyl alcohol) and chitosan-wrapped carbon nanotube. J. Membr. Sci. 2007, 300, 13–19. [Google Scholar] [CrossRef]
- Lu, L.Y.; Sun, H.L.; Peng, F.B.; Jiang, Z. Novel graphite-filled PVA/CS hybrid membrane for pervaporation of benzene/cyclohexane mixtures. J. Membr. Sci. 2006, 281, 245–252. [Google Scholar] [CrossRef]
- Khazaei, A.; Mohebbi, V.; Behbahani, R.M.; Ramazani, S.A. Pervaporation of toluene and iso-octane through poly(vinyl alcohol)/graphene oxide nanoplate mixed matrix membranes: Comparison of crosslinked and noncrosslinked membranes. J. Appl. Polym. Sci. 2018, 135, 45853. [Google Scholar] [CrossRef]
- Sun, H.; Lu, L.; Peng, F.; Wu, H.; Jiang, Z. Pervaporation of benzene/cyclohexane mixtures through CMS-filled poly(vinyl alcohol) membranes. Sep. Purif. Technol. 2006, 52, 203–208. [Google Scholar] [CrossRef]
- Vauclair, C.; Tarjus, H.; Schaetzel, P. Permselective properties of PVA-PAA blended membrane used for dehydration of fusel oil by pervaporation. J. Membr. Sci. 1997, 125, 293–301. [Google Scholar] [CrossRef]
- Park, H.C.; Meertens, R.M.; Mulder, M.H.V.; Smolders, C.A. Pervaporation of alcohol-toluene mixtures through polymer blend membranes of poly (acrylic acid) and poly (vinyl alcohol). J. Membr. Sci. 1994, 90, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Ping, Z.; Nguyen, Q.T.; Essamri, A.; Nee1, J. High-performance membranes for pervaporation II. Crosslinkedpoly(viny1 alcohol)—Poly(acry1ic acid) blends. Polym. Adv. Technol. 1994, 5, 320–326. [Google Scholar] [CrossRef]
- Hilmioglu, N.D.; Tulbentci, S. Pervaporative separation of isopropyl alcohol/water mixtures: Effects of the operation conditions. Desalin. Water Treat. 2012, 48, 191–198. [Google Scholar] [CrossRef]
- Hilmioglu, N.D.; Tulbentci, S. Separation of IPA/water mixtures by pervaporation: Sorption and pervaporation results. Vacuum 2004, 72, 35–40. [Google Scholar] [CrossRef]
- Inui, K.; Noguchi, T.; Miyata, T.; Uragami, T. Pervaporation characteristics of methyl-methacrylate-methacrylic acid copolymer membranes ionicallycrosslinked with metal ions for a benzene-cyclohexane mixture. J. Appl. Polym. Sci. 1999, 71, 233–241. [Google Scholar] [CrossRef]
- Matsui, S.; Paul, D.R. Pervaporation separation of aromatic/aliphatic hydrocarbons by crosslinked poly(methyl acrylate-co-acrylic acid) membranes. J. Membr. Sci. 2002, 195, 229–245. [Google Scholar] [CrossRef]
- Matsui, S.; Paul, D.R. Pervaporation separation of aromatic/aliphatic hydrocarbons by a series of ionicallycrosslinkedpoly(n-alkyl acrylate) membranes. J. Membr. Sci. 2003, 213, 67–83. [Google Scholar] [CrossRef]
- Ignat, M.; Farcas, A.; Vasilea, A.; Popovici, E. Calixarene-modified multi-wall carbon nanotubes, Zeolites and Related Materials: Trends, Targets and Challenges. In Proceedings of the 4th International FEZA Conference, online, 5–9 July 2021; Gédéon, A., Massiani, P., Babonneau, F., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2008; p. 389. [Google Scholar]
- Kononova, S.V.; Kremnev, R.V.; Suvorova, E.I.; Baklagina, Y.G.; Volchek, B.Z.; Uchytil, P.; Shabsels, B.M.; Romashkova, K.A.; Setnickova, K.; Reznickova, J. Pervaporation membranes with poly(γ-benzyl-l-glutamate) selective layers for separation of toluene-n-heptane mixtures. J. Membr. Sci. 2015, 477, 14–24. [Google Scholar] [CrossRef]
- Hyder, M.N.; Huang, R.Y.M.; Chen, P. Correlation of physicochemical characteristics with pervaporation performance of poly(vinyl alcohol) membranes. J. Membr. Sci. 2006, 283, 281–290. [Google Scholar] [CrossRef]
- Nurkeeva, Z.S.; Mun, G.A.; Dubolazov, A.V.; Khutoryanskiy, V.V. pH Effects on the Complexation, Miscibility and Radiation-Induced Crosslinking in Poly(acrylic acid)-Poly(vinyl alcohol) Blends. Macromol. Biosci. 2005, 5, 424–432. [Google Scholar] [CrossRef]
- Schwarz, H.H.; Malsch, G. Polyelectrolyte membranes for aromatic-aliphatic hydrocarbon separation by pervaporation. J. Membr. Sci. 2005, 247, 143–152. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Pinnau, I.; Freeman, B.D. (Eds.) Materials Science of Membranes for Gas and Vapor Separation; Wiley: Chichester, UK, 2006. [Google Scholar]
- Sakohara, S.; Koshi, T.; Asaeda, M. Separation of benzene/cyclohexane mixtures by dimethylaminoethyl methacrylate gel membranes formed in pores of a thin silica membrane. Kobunshi Ronbunshu 1995, 52, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Sakohara, S.; Koshi, T. Separation of benzene/cyclohexane mixtures by methacrylate gels formed in pores of a thin, porous ceramic membranes. Kobunshi Ronbunshu 1997, 54, 115–118. [Google Scholar] [CrossRef] [Green Version]
- Kononova, S.V.; Gubanova, G.N.; Korytkova, E.N.; Sapegin, D.A.; Setnickova, K.; Petrychkovych, R.; Uchytil, P. Polymer Nanocomposite Membranes. Appl. Sci. 2018, 8, 1181. [Google Scholar] [CrossRef]















| Polymer | Glass Transition Temperature (DSC Data), °C |
|---|---|
| PVA | 78.0 |
| PAA | 128.0 |
| Composition 1 (20 wt.% PAA) | 75.4; 111.4 |
| Composition 2 (50 wt.% PAA) | 115.2 |
| Composition 3 (20 wt.% PAA) | 106.7 |
| Composition 4 (50 wt.% PAA) | 112.3 |
| N | Membrane | Components of the Feed Mixture | T, °C | The Amount of Toluene, wt.% | Refs | |
|---|---|---|---|---|---|---|
| Feed Mixture | Permeate | |||||
| 1 | Poly(oxyethylene methacrylate) | Toluene—n-heptane | 80 | 21 | 56 | [47] |
| 2 | PVA-PAA (10–40 wt.% PVA) | methanol-toluene | 25 | 29 | ~5 | [36] |
| 3 | PVA-PAA (80 wt.% PVA) Composition 1 | Toluene—n-heptane | 40 | 50 | 68 | “this work” |
| 4 | PVA-PAA (80 wt.% PVA) Composition 1 | methanol-toluene | 40 | 29 | 2.5 | “this work” |
| Membrane, N | Composition of the Feed Mixture, wt.% | T, °C | Permeate, C(methanol), wt.% | J, kg m−2 h−1 | f | Number of Coating Procedures | Post—Treatment, T, °C | |
|---|---|---|---|---|---|---|---|---|
| Methanol | Toluene | |||||||
| 1 | 100 | 0 | 22 | 100 | 0.28 ± 0.01 | - | 1 | - |
| 1 | 70 | 30 | 22 | 78.70 | 2.25 ± 0.11 | 1.58 | 1 | - |
| 1 | 50 | 50 | 22 | 56.35 | 0.92 ± 0.05 | 1.29 | 1 | - |
| 1 | 0 | 100 | 22 | 0 | 0.020 ± 0.001 | - | 1 | - |
| 2 | 71 | 29 | 22 | 99.07 | 0.080 ± 0.004 | 21.29 | 2 | - |
| 2 | 71 | 29 | 40 | 97.43 | 0.120 ± 0.006 | 10.79 | 2 | - |
| 2 | 67 | 33 | 40 | 97.82 | 0.59 ± 0.03 | 12.95 | 2 | - |
| 3 | 71 | 29 | 22 | 97.72 | 0.180 ± 0.009 | 12.95 | 1 | 110 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kononova, S.V.; Kremnev, R.V.; Gubanova, G.N.; Vlasova, E.N.; Popova, E.N.; Vylegzhanina, M.E.; Volkov, A.Y. Effect of Phase Heterogeneity on the Properties of Poly(vinyl alcohol)-Based Composite Pervaporation Membranes. Membranes 2022, 12, 1185. https://doi.org/10.3390/membranes12121185
Kononova SV, Kremnev RV, Gubanova GN, Vlasova EN, Popova EN, Vylegzhanina ME, Volkov AY. Effect of Phase Heterogeneity on the Properties of Poly(vinyl alcohol)-Based Composite Pervaporation Membranes. Membranes. 2022; 12(12):1185. https://doi.org/10.3390/membranes12121185
Chicago/Turabian StyleKononova, Svetlana V., Roman V. Kremnev, Galina N. Gubanova, Elena N. Vlasova, Elena N. Popova, Milana E. Vylegzhanina, and Anatoly Ya. Volkov. 2022. "Effect of Phase Heterogeneity on the Properties of Poly(vinyl alcohol)-Based Composite Pervaporation Membranes" Membranes 12, no. 12: 1185. https://doi.org/10.3390/membranes12121185