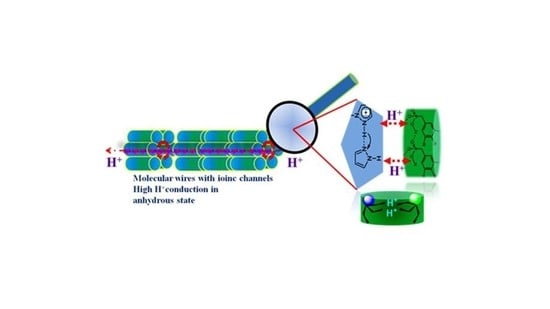
Proton Conducting Membranes with Molecular Self Assemblies and Ionic Channels for Efficient Proton Conduction
Abstract
:1. Introduction
2. Result and Discussion
3. Conclusions
4. Experimental
4.1. Materials
4.2. Film or Membrane Formation on the Substrate
4.3. Proton (Anhydrous) Conductivity Measurements
4.4. Thermogravimetric Analysis (TGA)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2DWAXS | 2-Dimensional Wide Angle Scatterings |
| TGA | Thermogravimetric analysis |
| FT-IR | Fourier-transform infrared |
References
- Frieden, E. Non-covalent interactions: Key to biological flexibility and specificity. J. Chem. Educ. 1975, 52, 754. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498. [Google Scholar] [CrossRef] [Green Version]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18. [Google Scholar] [CrossRef]
- Knowles, R.R.; Jacobsen, E.N. Attractive noncovalent interactions in asymmetric catalysis: Links between enzymes and small molecule catalysts. Proc. Natl. Acad. Sci. USA 2010, 107, 20678. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Dong, J.; Viviani, M.; Tulini, I.; Pontillo, N.; Maity, S.; Zhou, Y.; Roos, W.H.; Liu, K.; Herrmann, A.; et al. De novo rational design of a freestanding, supercharged polypeptide, proton-conducting membrane. Sci. Adv. 2020, 6, 29. [Google Scholar] [CrossRef]
- Yamada, M.; Sugihara, T.; Yamada, T. Anhydrous proton-conducting material consisting of basic protein protamine. J. Electroanal. Chem. 2021, 897, 115586. [Google Scholar] [CrossRef]
- Coropceanu, V.; Cornil, J.; da Silva Filho, D.A.; Olivier, Y.; Silbey, R.; Brédas, J.L. Charge Transport in Organic Semiconductors. Chem. Rev. 2007, 107, 926. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Stimuli directed alignment of self-organized one-dimensional semiconducting columnar liquid crystal nanostructures for organic electronics. Prog. Mater. Sci. 2019, 104, 1–52. [Google Scholar] [CrossRef]
- Allen, F.I.; Comolli, L.R.; Kusoglu, A.; Modestino, M.A.; Minor, A.M.; Weber, A.Z. Weber Morphology of Hydrated As-Cast Nafion Revealed through Cryo Electron Tomography. ACS Macro Lett. 2015, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Orfino, F.; Holdcroft, S. The morphology of Nafion: Are ion clusters bridged by channels or single ionic sites? J. New Mater. Electrochem. Syst. 2000, 3, 4. [Google Scholar]
- Su, Z.; Huang, M.; Cheng, S.Z. Complex self-assembled lattices from simple polymer blends. Proc. Natl. Acad. Sci. USA 2020, 117, 19618–19620. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, X.; Cheng, S.Z.D. Reaction: Precision Macromolecules for Self-Assembly. Chem 2019, 5, 492–493. [Google Scholar] [CrossRef] [Green Version]
- Sasselli, I.R.; Syrgiannis, Z. Small Molecules Organic Co-Assemblies as Functional Nanomaterials. Eur. J. Org. Chem. 2020, 33, 5305–5318. [Google Scholar] [CrossRef]
- Palmer, L.C.; Stupp, S.I. Molecular Self-Assembly into One-Dimensional Nanostructures. Acc. Chem. Res. 2008, 41, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Pisula, W.; Sieber, C.; Klapper, M.; Müllen, K. Anhydrous proton conduction in self-assembled and disassembled ionic molecules. J. Mater. Chem. A 2018, 6, 6074–6084. [Google Scholar] [CrossRef]
- Kumar, A.; Pisula, W.; Müllen, K. One Dimensional Enhanced Anhydrous Proton Conduction in Well Defined Molecular Columns Induced by Non-Covalent Interactions. ChemPhysChem 2019, 20, 651–654. [Google Scholar] [CrossRef]
- Chen, Y.; Thorn, M.; Christensen, S.; Versek, C.; Poe, A.; Hayward, R.C.; Tuominen, M.T.; Thayumanavan, S. Thayumanavan Enhancement of anhydrous proton transport by supramolecular nanochannels in comb polymers. Nat. Chem. 2010, 2, 503–508. [Google Scholar] [CrossRef]
- Widelicka, M.; Pogorzelec-Glaser, K.; Pietraszko, A.; Ławniczak, P.; Pankiewicz, R.; Łapiński, A. Order–disorder phase transition in an anhydrous pyrazole-based proton conductor: The enhancement of electrical transport properties. Phys. Chem. Chem. Phys. 2017, 19, 25653–25661. [Google Scholar] [CrossRef] [Green Version]
- Umeyama, D.; Horike, S.; Inukai, M.; Kitagawa, S. Integration of Intrinsic Proton Conduction and Guest-Accessible Nanospace into a Coordination Polymer. J. Am. Chem. Soc. 2013, 135, 11345–11350. [Google Scholar] [CrossRef]
- Nagamani, C.; Versek, C.; Thorn, M.; Tuominen, M.T.; Thayumanavan, S. Proton conduction in 1H-1,2,3-triazole polymers: Imidazole-like or pyrazole-like? J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1851–1858. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton Conductivity: Materials and Applications. Chem. Mater. 1996, 8, 610–641. [Google Scholar] [CrossRef]
- Kawahara, M.; Morita, J.; Rikukawa, M.; Sanui, K.; Ogata, N. Synthesis and proton conductivity of thermally stable polymer electrolyte: Poly(benzimidazole) complexes with strong acid molecules. Electrochim. Acta 2000, 45, 1395. [Google Scholar] [CrossRef]
- Bouchet, R.; Siebert, E. Proton conduction in acid doped polybenzimidazole. Solid State Ion. 1999, 118, 287. [Google Scholar] [CrossRef]
- Kumar, A. Cooperative proton conduction in sulfonated and phosphonated hybrid random copolymers. J. Mater. Chem. A 2020, 8, 22632–22636. [Google Scholar] [CrossRef]
- Kumar, A.; Pisula, W.; Markova, D.; Klapper, M.; Müllen, K. Proton-Conducting Poly(phenylene oxide)–Poly(vinyl benzyl phosphonic acid) Block Copolymers via Atom Transfer Radical Polymerization. Macro. Chem. Phy. 2012, 213, 489–499. [Google Scholar] [CrossRef]
- Markova, D.; Kumar, A.; Klapper, M.; Müllen, K. Phosphonic acid-containing homo-, AB and BAB block copolymers via ATRP designed for fuel cell applications. Polymer 2009, 50, 3411–3421. [Google Scholar] [CrossRef]
- Catalan, J.; Elguero, J. Basicity and Acidity of Azoles. Adv. Heterocycl. Chem. 1987, 41, 187. [Google Scholar]
- Blumenfeld, A.L.; Golub, A.S.; Protsenko, G.; Novikov, Y.N.; Casciola, M.; Costantino, M.U. Costantino NMR investigation on molecular mobility of pyrazole and pyridazineintercalated in layered α-zirconium phosphate. Solid State Ion. 1994, 68, 105–110. [Google Scholar] [CrossRef]
- Kawada, A.R.; McGhie, M.M. Labes Protonic Conductivity in Imidazole Single Crystal. J. Chem. Phys. 1970, 52, 3121. [Google Scholar] [CrossRef]
- Hassanali, A.; Giberti, F.; Cuny, J.; Kühne, T.D.; Parrinello, M. Proton transfer through the water gossamer. Proc. Natl. Acad. Sci. USA 2013, 110, 13723–13728. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, P.; Hao, L.; Cheng, P.; Chen, Y.; Zhang, Z. Grotthuss Proton-Conductive Covalent Organic Frameworks for Efficient Proton Pseudocapacitors. Angew. Chem. 2021, 60, 22009–22016. [Google Scholar] [CrossRef]
- Dai, Q.; Liu, Z.; Huang, L.; Wang, C.; Zhao, Y.; Fu, Q.; Zheng, A.; Zhang, H.; Li, X. Thin-film composite membrane breaking the trade-off between conductivity and selectivity for a flow battery. Nat. Commun. 2020, 11, 13. [Google Scholar] [CrossRef]
- Kim, O.; Kim, K.; Choi, U.H.; Park, M.J. Tuning anhydrous proton conduction in single-ion polymers by crystalline ion channels. Nat. Commun. 2018, 9, 5029. [Google Scholar] [CrossRef] [Green Version]
- Jangu, C.; Wang, J.H.; Wang, D.; Fahs, G.; Heflin, J.R.; Moore, R.B.; Colby, R.H.; Long, T.E. Imidazole-containing triblock copolmers with a synergy of ether and imidazolium sites. J. Mater. Chem. C 2015, 3, 3891–3901. [Google Scholar] [CrossRef] [Green Version]
- Hogberg, D.; Soberats, B.; Uchida, S.; Yoshio, M.; Kloo, L.; Segawa, H.; Kato, T. Nanostructured Two-Component Liquid-Crystalline Electrolytes for High-Temperature Dye-Sensitized Solar Cells. Chem. Mater. 2014, 26, 6496–6502. [Google Scholar] [CrossRef]
- Yin, C.; Li, J.; Zhou, Y.; Zhang, H.; Fang, P.; He, C. Enhancement in Proton Conductivity and Thermal Stability in Nafion Membranes Induced by Incorporation of Sulfonated Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 14026–14035. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, J.; Tang, Z.; Li, Y.; Lin, J. High-temperature proton exchange membranes from ionic liquid absorbed/doped superabsorbents. J. Mater. Chem. 2012, 22, 15836–15844. [Google Scholar] [CrossRef]
- Chikashige, Y.; Chikyu, Y.; Miyatake, K.; Watanabe, M. Poly(arylene ether) ionomers containing sulfofluorenyl groups for fuel cell applications. Macromolecules 2005, 38, 7121–7126. [Google Scholar] [CrossRef]
- Kundu, S.; Simon, L.C.; Fowler, M.; Grot, S. Mechanical properties of Nafion™ electrolytic membranes under hydrated conditions. Polymer 2005, 46, 11707–11715. [Google Scholar] [CrossRef]
- Yasuda, T.; Miyatake, K.; Hirai, M.; Nanasawa, M.; Watanabe, M. Synthesis and properties of polyimide ionomers containing sulfoalkoxy and fluorenyl groups. J. Polym. Sci. A Polym. Chem. 2005, 43, 4439–4445. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, T.; Gao, G.; Zhang, X.; Wei, W.; Liu, W.; Ding, S. Highly stretchable and transparent ionic conducting elastomers. Nat. Comm. 2018, 9, 2630. [Google Scholar] [CrossRef] [Green Version]
- Marani, D.; Di Vona, M.L.; Traversa, E.; Licoccia, S.; Beurroies, I.; Llewellyn, P.L.; Knauth, P. Thermal Stability and Thermodynamic Properties of Hybrid Proton-Conducting Polyaryl Etherketones. J. Phys. Chem. B 2006, 110, 15817–15823. [Google Scholar] [CrossRef]





| Molecular Wires | Anhydrous Proton Conductivity | Activation Energy (Ea) | |
|---|---|---|---|
| 1 | Pure compound 1 | 8 × 10−7 S/cm | 0.34 eV |
| 2 | Compound 1/Pyrazole | 2 × 10−3 S/cm | 0.12 eV |
| 3 | Compound 1/Triazole | 1 × 10−3 S/cm | 0.41 eV |
| 4 | Compound 1/Benzimidazole | 5 × 10−2 S/cm | 0.51 eV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Chang, D.W. Proton Conducting Membranes with Molecular Self Assemblies and Ionic Channels for Efficient Proton Conduction. Membranes 2022, 12, 1174. https://doi.org/10.3390/membranes12121174
Kumar A, Chang DW. Proton Conducting Membranes with Molecular Self Assemblies and Ionic Channels for Efficient Proton Conduction. Membranes. 2022; 12(12):1174. https://doi.org/10.3390/membranes12121174
Chicago/Turabian StyleKumar, Avneesh, and Dong Wook Chang. 2022. "Proton Conducting Membranes with Molecular Self Assemblies and Ionic Channels for Efficient Proton Conduction" Membranes 12, no. 12: 1174. https://doi.org/10.3390/membranes12121174