Transport of Heavy Metals Pb(II), Zn(II), and Cd(II) Ions across CTA Polymer Membranes Containing Alkyl-Triazole as Ions Carrier
Abstract
:1. Introduction
2. Experimental
2.1. Reagents

2.2. Equipment
2.3. Polymer Inclusion Membrane Preparation
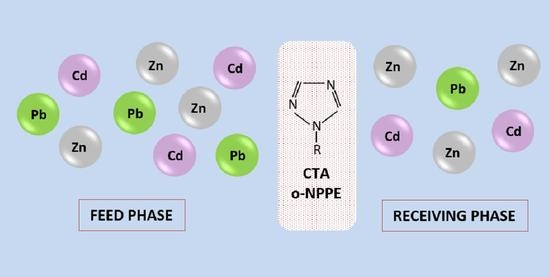
2.4. Parameters Characterizing the Transport Process
3. Results and Discussion
3.1. Membrane Characteristics
3.1.1. Thermal Stability of PIM with 1-Alkyltriazole
3.1.2. FT-IR Analysis of the PIM with 1-Hexyltriazole
3.2. Separation of Zn(II) from Zn(II)-Cd(II)-Pb(II) Mixture
- Transport:
- 2.
- Back-transport:
3.3. Membrane Diffusion Coefficients of Zn(II), Cd(II), and Pb(II) Ions across PIMs with 1- Alkyltriazole
3.4. Recovery of Metal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2017, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology, Volume 3: Environmental Toxicology; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.K.; Chen, J.P.; Hung, Y.-T.; Shammas, N. Heavy Metals in the Environment; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Fenech, M. The in vivo micronucleus technique. Mutat. Res. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Blais, J.F.; Djedidi, Z.; Cheikh, R.B.; Tyagi, R.D.; Mercier, G. Metals Precipitation from Effluents: Review. Practice Periodical of Hazardous, Toxic, and Radioactive. Waste Manag. 2008, 12, 135–149. [Google Scholar] [CrossRef]
- Verbych, S.; Hilal, N.; Sorokin, G.; Leaper, M. Ion Exchange Extraction of Heavy Metal Ions from Wastewater. Sep. Sci Technol. 2005, 39, 2031–2040. [Google Scholar] [CrossRef]
- Taseidifar, M.; Makavipour, F.; Pashley, R.M.; Rahman, A.F.M.M. Removal of heavy metal ions from water using ion flotation. Environ. Technol. Innov. 2017, 8, 182–190. [Google Scholar] [CrossRef]
- Dąbrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Zulkefeli, N.S.W.; Weng, S.K.; Halim, N.S.A. Removal of Heavy Metals by Polymer Inclusion Membranes. Curr. Pollut. Rep. 2018, 4, 84–92. [Google Scholar] [CrossRef]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.; Yang, Y.; Bartsch, R.A. Heavy metal separation with polymer inclusion membranes. J. Membr. Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Yildiz, Y.; Manzak, A.; Aydýn, B.; Tutkun, O. Preparation and application of polymer inclusion membranes (PIMs) including alamine 336 for the extraction of metals from an aqueous solution. Mater. Technol. 2014, 48, 791–796. [Google Scholar]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Walkowiak, W.; Bartsch, R.A.; Kozłowski, C.; Gęga, J.; Charewicz, W.; Amiri-Elias, B. Separation and removal of metal ionic species by polymer inclusion membranes. J. Radioanal. Nucl. Chem. 2000, 246, 643–650. [Google Scholar] [CrossRef]
- Almeida, I.M.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis—A review. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Mornane, P.; Potter, L.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Application, 3rd ed.; John Willey & Sons: Newark, CA, USA, 2004; ISBN 9780470743720. [Google Scholar]
- Pereira, N.; St John, A.; Cattrall, R.W.; Perera, J.M.; Kolev, S.D. Influence of the composition of polymer inclusion membranes on their homogeneity and flexibility. Desalination 2009, 236, 327–333. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of Hydrophobic Alkylimidazoles in the Separation of Non-Ferrous Metal Ions across Plasticised Membranes—A Review. Membranes 2020, 10, 331. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kurdziel, K.; Gabryszewski, M. Stability and structure of transition metal complexes with azoles in aqueous solution-XXII. Complexing behaviour of 1,2,4-triazole, 3-amino-1,2,4-triazole and 4-amino-1,2,4-triazole. J. Inorg. Nucl. Chem. 1980, 42, 587–592. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Lenarcik, B. Determintion of stability constants of Cu(II) complexes with 1-alkyl-1,2,4-triazoles by liquid-liquid partition method. In Proceedings of the XIX-th International Symposium on Physicochemical Methods of Separation, “Ars Separatoria 2004”, Złoty Potok, Czestochowa, Poland, 2–5 June 2004; pp. 236–238. [Google Scholar]
- Lenarcik, B.; Rauckyte, T.; Kopkowski, A. Application of extraction method to the investigation of the stability constants of Ni(II) complexes with 1-alkyl-1,2,4-triazoles by using several organic solvents. In Proceedings of the XVII International Symposium on Physicochemical Methods of Separation, “Ars Separatoria 2002”, Borówno, Poland, 17–20 June 2002. [Google Scholar]
- Naseem, R.; Tahir, S.S. Removal of Pb(II) from aqueous solution by using bentonite as an adsorbent. Water Res. 2001, 35, 3982–3986. [Google Scholar] [CrossRef]
- Sari, A.; Tuzen, M. Biosorption of Pb and Cd from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef]
- Barbier, F.; Duc, G.; Petit-Ramel, M. Adsorption of lead and cadmium ions from aqueous solution to the montmorillonite: Water interface. Colloids Surf. A Phys. Eng. Asp. 2000, 166, 153–159. [Google Scholar] [CrossRef]
- Selatnia, A.; Boukazoula, A.; Kechid, N.; Bakhti, M.Z. Chergui, A.; Kerchich Y. Biosorption of lead(II) from aqueous solution by a bacterial dead Streptomyces rimosus biomass. Biochem. Eng. J. 2004, 19, 127–135. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Urbaniak, W. Cadmium(II) and lead(II) extraction and transport through polymer inclusion membranes with 1-alkylimidazole. Desalin. Water Treat. 2021, 214, 56–63. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M.; Pyszka, I. Application of Polymer Inclusion Membranes Doped with Alkylimidazole to Separation of Silver and Zinc Ions from Model Solutions and after Battery Leaching. Materials 2020, 13, 3103. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I. New polymer inclusion membrane in separation of nonferrous metal ions from aqueous solutions. Membranes 2020, 10, 385. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The Application of Polymer Inclusion Membranes Based on CTA with 1-alkylimidazole for the Separation of Zinc(II) and Manganese(II) Ions from Aqueous Solutions. Polymers 2019, 11, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Polymer Inclusion Membranes (PIMs) doped with Alkylimidazole and their Application in the Separation of Non-Ferrous Metal Ions. Polymers 2019, 11, 1780. [Google Scholar] [CrossRef] [Green Version]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Urbaniak, W. New Polymer Inclusion Membranes in the Separation of Palladium, Zinc and Nickel Ions from Aqueous Solutions. Polymers 2021, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Kislik, V.S. Liquid Membrane. Principles & Applications in Chemical Separations & Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- St John, A.M.; Cattrall, R.W.; Kolev, S.D. Determination of the initial flux of polymer inclusion membranes. Sep. Purif. Technol. 2013, 116, 41–45. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Bautista-Flores, A.N.; San Miguel, E.R.; Muhammed, M.; Gyves, J. Transport characterization of a PIM system used for the extraction of Pb(II) using D2EHPA as carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Ulewicz, M.; Szczygelska-Tao, J.; Biernat, J.F. Selectivity of Pb(II) transport across polymer inclusion membranes doped with imidazole azothiacrown ethers. J. Membr. Sci. 2009, 344, 32–38. [Google Scholar] [CrossRef]
- Arous, O.; Amara, M.; Kerdjoudj, H. Synthesis and characterization of cellulose triacetate and poly(ethylene imine) membranes containing a polyether macrobicyclic: Their application to the separation of copper(II) and silver(I) ions. J. Appl. Polymer Sci. 2004, 93, 1401–1410. [Google Scholar] [CrossRef]
- Arous, O.; Kerdjoudj, H.; Seta, P. Comparison of carrier-facilitated silver(I) and copper(II) ions transport mechanisms in a supported and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P.; Drioli, E. Fixed sites plasticized cellulose triacetate membranes containing crown ethers for silver(I), copper(II) and gold(III) ions transport. J. Membr. Sci. 2004, 228, 149–157. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of Metal Species by Supported Liquid Membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Ulewicz, M. Separation of Zn(II), Cr(III), and Ni(II) ions using the polymer inclusion membranes containing acetylacetone derivative as the carrier. Membranes 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Lenarcik, B.; Kierzkowska, A. The Influence of Alkyl Chain Length and Steric Effect on Extraction of Zinc(II) Complexes with 1-Alkyl-2-methylimidazoles. Solv. Ext. Ion Exch. 2006, 24, 433–445. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of solvatation of ions. J.Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Tor, A.; Arslan, G.; Muslu, H.; Celikas, A.; Cengeloglu, Y.; Ersoz, M. Facilitated transport of Cr(III) thought polymer inclusion membrane with di(2-ethylhexyl)phosphoric acid (DEHPA). J. Membr. Sci. 2009, 329, 169–174. [Google Scholar] [CrossRef]








 | No. | R = | Compound | Boiling Point, °C, at Pressure 1 hPa |
| 1 | –C6H13 | 1-hexyl-1,2,4-triazole | 179–181 | |
| 2 | –C8H17 | 1-octyl-1,2,4-triazole | 185–188 | |
| 3 | –C10H21 | 1-decyl-1,2,4-triazole | 216–218 |
| Polymer Inclusion Membranes with 1-Alkyl-Triazole | |||
|---|---|---|---|
| Carrier | 1 | 2 | 3 |
| Roughness, nm | 4.52 | 4.89 | 5.21 |
| The Composition of Membrane | The First Step | The Second Step | ||
|---|---|---|---|---|
| Temp. °C | Weight Loss, % | Temp. °C | Weight Loss, % | |
| CTA-o-NPPE | 277.0 | 80.61 | 367.8 | 16.37 |
| CTA-o-NPPE– 1-hexyltriazole before process | 268.0 | 28.20 | 343.4 | 44.33 |
| CTA-o-NPPE– 1-hexyltriazole after process | 249.8 | 44.91 | 359.6 | 44.33 |
| Range of Wavenumbers, cm−1 | Indicated Bonds |
|---|---|
| 2925–2750 | C-H, N-H, O-H |
| 1755–1450 | C-C, C-O, C-N |
| 1660–1490; 1390–1260 | N-O |
| 1635–1470 | C=C, C=N (triazole ring) |
| 870–840 | C-N |
| 770–665 | C-H |
| 480–390 | -NO2 |
| Carrier | Metal Ions | J0, μmolm−2·s−1 | Selectivity Orders and Selectivity Coefficients SZn(II)/M(II) SPd(II)/M(II) |
|---|---|---|---|
| 1 | Zn(II) | 7.62 | Zn(II) > Cd(II) > Pb(II) 1.8 16.2 |
| Cd(II) | 4.25 | ||
| Pb(II) | 0.47 | ||
| 2 | Zn(II) | 9.05 | Zn(II) > Cd(II) > Pb(II) 2.0 9.5 |
| Cd(II) | 4.58 | ||
| Pb(II) | 0.95 | ||
| 3 | Zn(II) | 12.34 | Zn(II) > Cd(II) > Pb(II) 2.4 8.8 |
| Cd(II) | 5.19 | ||
| Pb(II) | 1.41 |
| Carrier | Metal Ion | Δo, s/m | Do, cm2/s |
|---|---|---|---|
| 1 | Zn(II) | 103.04 | 2.38 × 10−7 |
| Cd(II) | 176.08 | 3.52 × 10−8 | |
| Pb(II) | 1256.14 | 2.73 × 10−11 | |
| 2 | Zn(II) | 128.25 | 2.79 × 10−7 |
| Cd(II) | 196.13 | 3.86 × 10−8 | |
| Pb(II) | 1287.06 | 2.91 × 10−11 | |
| 3 | Zn(II) | 135.42 | 2.94 × 10−7 |
| Cd(II) | 216.17 | 3.95 × 10−8 | |
| Pb(II) | 1298.24 | 3.12 × 10−11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzymińska-Lenarcik, E.; Kwiatkowska-Marks, S.; Kościuszko, A. Transport of Heavy Metals Pb(II), Zn(II), and Cd(II) Ions across CTA Polymer Membranes Containing Alkyl-Triazole as Ions Carrier. Membranes 2022, 12, 1068. https://doi.org/10.3390/membranes12111068
Radzymińska-Lenarcik E, Kwiatkowska-Marks S, Kościuszko A. Transport of Heavy Metals Pb(II), Zn(II), and Cd(II) Ions across CTA Polymer Membranes Containing Alkyl-Triazole as Ions Carrier. Membranes. 2022; 12(11):1068. https://doi.org/10.3390/membranes12111068
Chicago/Turabian StyleRadzymińska-Lenarcik, Elżbieta, Sylwia Kwiatkowska-Marks, and Artur Kościuszko. 2022. "Transport of Heavy Metals Pb(II), Zn(II), and Cd(II) Ions across CTA Polymer Membranes Containing Alkyl-Triazole as Ions Carrier" Membranes 12, no. 11: 1068. https://doi.org/10.3390/membranes12111068