Study on the Preparation of Cellulose Acetate Separation Membrane and New Adjusting Method of Pore Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
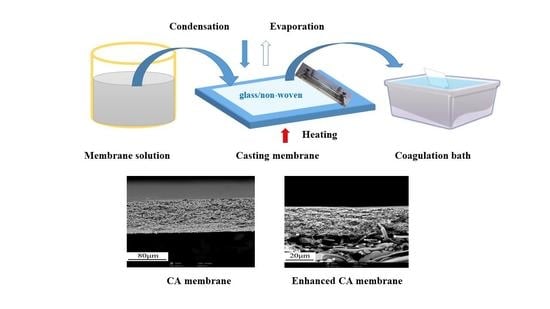
2.2. Membrane Fabrication
2.3. Characterization of the CA Membranes
2.3.1. Morphology Observation
2.3.2. Infrared Spectra Measurements (FTIR)
2.3.3. Permeation Characterization
2.3.4. Pore Sizes Analysis
2.3.5. Porosity Analysis
2.3.6. Viscosity Test
3. Results
3.1. Effect of Compositions of Casting Solution on Membrane Properties
3.1.1. Effect of CA Content on Membrane Structure and Performance
3.1.2. Effect of Acetone/Solvent Ratio on Membrane Structure and Performance
3.1.3. Effect of Glycerol/CA Ratio on Membrane Structure and Performance
3.2. Effect of Treatment Conditions on Membrane Properties
3.2.1. Effect of Evaporation Temperature on Membrane Structure and Performance
3.2.2. Effect of Evaporation Time on Membrane Structure and Performance
3.2.3. Effect of Ambient Humidity on Membrane Structure and Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jazini, F.; Karimi, M.; Azari, S. Tuning the pore features of cellulose acetate/cellulose triacetate membranes via post-casting solvent treatment for forward osmosis. Carbohydr. Polym. 2021, 255, 117348. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.C.; Pereira, J.C.; Almeida, N.; Marques, P.; Faria, M.; Gonçalves, M.C. Improving hydraulic permeability, mechanical properties, and chemical functionality of cellulose acetate-based membranes by co-polymerization with tetraethyl orthosilicate and 3-(aminopropyl)triethoxysilane. Carbohydr. Polym. 2021, 261, 117813. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Doherty, C.M.; Ricci, E.; Chen, G.Q.; Angelis, M.G.D.; Kentish, S.E. The influence of propane and n-butane on the structure and separation performance of cellulose acetate membranes. J. Membr. Sci. 2021, 638, 119677. [Google Scholar] [CrossRef]
- Li, D.F.; Chung, T.S.; Wang, R. Morphological aspects and structure control of dual-layer asymmetric hollow fiber membranes formed by a simultaneous co-extrusion approach. J. Membr. Sci. 2004, 243, 155–175. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.B. An emerging Pore-Making strategy: Confined swelling-induced pore generation in block copolymer materials. Adv. Mater. 2011, 23, 2134–2148. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, P.B.; Zhao, Y.F.; Zhu, L.P.; Zhu, B.K.; Xu, Y.Y. Preparation and characterization of poly (N-vinyl imidazole) gel-filled nanofiltration membranes. J. Membr. Sci. 2015, 492, 380–391. [Google Scholar] [CrossRef]
- Cheng, L.; Zhu, L.P.; Zhang, P.B.; Sun, J.; Zhu, B.K.; Xu, Y.Y. Molecular separation by poly (N-vinyl imidazole) gel-filled membranes. J. Membr. Sci. 2016, 497, 472–484. [Google Scholar] [CrossRef]
- Athira, V.B.; Mohanty, S.; Nayak, S.K. Preparation and characterization of porous polyethersulfone (PES) membranes with improved biocompatibility by blending sulfonated polyethersulfone (SPES) and cellulose acetate (CA)—A comparative study. Mater. Today Commun. 2020, 25, 101544. [Google Scholar]
- Liu, T.Y.; Li, C.K.; Pang, B.; Bruggen, B.V.; Wang, X.L. Fabrication of a dual-layer (CA/PVDF) hollow fiber membrane for RO concentrate treatment. Desalination 2015, 365, 57–69. [Google Scholar] [CrossRef]
- Silva, M.A.; Felgueiras, H.P.; de Amorim, M.T.P. Carbon based membranes with modified properties: Thermal, morphological, mechanical and antimicrobial. Cellulose 2020, 27, 1497–1516. [Google Scholar] [CrossRef]
- Matsuyama, H.; Ohga, K.; Maki, T.; Tearamoto, M.; Nakatsuka, S. Porous cellulose acetate membrane prepared by Thermally induced phase separation. J. Appl. Polym. Sci. 2003, 89, 3951–3955. [Google Scholar] [CrossRef]
- Blachechen, L.S.; Souza, M.A.; Petri, D.F.S. Effect of humidity and solvent vapor phase on cellulose esters films. Cellulose 2012, 19, 443–457. [Google Scholar] [CrossRef]
- Lee, W.G.; Kim, D.Y.; Kang, S.W. Porouscellulose acetate by specific solvents with water pressure treatment for applications to separator and membranes. Macromol. Res. 2018, 26, 630–633. [Google Scholar] [CrossRef]
- Nguyen, T.P.N.; Yun, E.T.; Kim, I.C.; Kwon, Y.N. Preparation of cellulose triacetate/cellulose acetate (CTA/CA)-based membranes for forward osmosis. J. Membr. Sci. 2013, 433, 49–59. [Google Scholar] [CrossRef]
- Ali, A.; Yunus, R.M.; Awang, M.; Mat, R. The effects of evaporation time on morphological structure of polysulfone/cellulose acetate phthalate/polyvinylpyrrolidone (PSf/CAP/PVP) blend membranes. Appl. Mech. Mater. 2015, 695, 77–80. [Google Scholar] [CrossRef]
- Nulens, Y.L.; Verbeke, R.; Mariën, H.; Koschine, T.; Dickmann, M.; Egger, W.; Vankelecom, I.F.J. Tuning the porosity of asymmetric membranes via simple post-synthesis solvent-treatment for non-aqueous applications. Sep. Purif. Technol. 2019, 217, 147–153. [Google Scholar]
- Kima, H.Y.; Chob, Y.; Kanga, S.W. Porous cellulose acetate membranes prepared by water pressure-assisted process for water-treatment. J. Ind. Eng. Chem. 2019, 78, 421–424. [Google Scholar] [CrossRef]







| Preparation Methods | Technical Features | Membrane Characteristics |
|---|---|---|
| Non-solvent induced phase separation (NIPS) | Simple equipment requirements; No high temperature and high pressure; Multiple influencing factors | Dense layer and macroporous structure; low mechanical strength |
| The thermally induced phase separation (TIPS) | High equipment requirements; High energy consumption | High porosity; high flux; good mechanical strength |
| Volatilize induced phase separation (VIPS) | Normal temperature and pressure conditions; High humidity and clean steam displacement | Porous membrane structure, large flux; easy to control surface structure |
| Vapor-assisted nonsolvent induced phase separation (VNIPS) | Composite technology; Specific humidity conditions | Uniform and controllable separation of membrane holes; high porosity; high flux |
| NO | Temperature (°C) | Humidity (%) | Evaporation Time (S) | Bath Temperature (°C) |
|---|---|---|---|---|
| a | 25.0 | ≥90 | 120 | 25.0 |
| b | 55.0 | ≥90 | 120 | 25.0 |
| c | 100.0 | ≥90 | 120 | 25.0 |
| d | 150.0 | ≥90 | 120 | 25.0 |
| e | 100.0 | ≥90 | 10 | 25.0 |
| f | 100.0 | ≥90 | 30 | 25.0 |
| g | 100.0 | ≥90 | 60 | 25.0 |
| h | 100.0 | ≥90 | 120 | 25.0 |
| i | 55.0 | 30 | 120 | 25.0 |
| j | 55.0 | 60 | 120 | 25.0 |
| k | 55.0 | ≥90 | 120 | 25.0 |
| NO | CA% | Flux (L·m−2·h−1·bar−1) | Bubble Point Pressure (MPa) | Maximum Pore Size (μm) | Porosity (%) | Viscosity (mPa·s) |
|---|---|---|---|---|---|---|
| 1 | 7.0% | 14,274.1 ± 485.1 | 0.154 ± 0.004 | 1.87 | 94.7 ± 3.2 | 750 |
| 2 | 8.0% | 8942.9 ± 234.5 | 0.225 ± 0.006 | 1.28 | 92.7 ± 2.5 | 1310 |
| 3 | 9.0% | 1438.5 ± 43.1 | 0.314 ± 0.010 | 0.92 | 88.2 ± 4.5 | 2410 |
| 4 | 10.0% | 181.5 ± 10.2 | 0.364 ± 0.015 | 0.79 | 87.5 ± 5.2 | 3530 |
| NO | Acetone/DMAc | Flux (L·m−2·h−1·bar−1) | Bubble Point Pressure (MPa) | Maximum Pore Size (μm) | Porosity (%) | Viscosity (mPa·s) |
|---|---|---|---|---|---|---|
| 5 | 0 | 4320.0 ± 34.5 | 0.270 ± 0.010 | 1.07 | 93.0 ± 3.1 | 2415.5 |
| 6 | 0.25 | 13,012.7 ± 435.1 | 0.185 ± 0.004 | 1.56 | 92.2 ± 1.3 | 1447.5 |
| 7 | 0.50 | 13,671.2 ± 345.2 | 0.215 ± 0.009 | 1.34 | 91.7 ± 2.0 | 1250.5 |
| 8 | 0.75 | 431.7 ± 10.2 | 0.203 ± 0.006 | 1.42 | 85.4 ± 0.6 | 987.5 |
| NO | Glycerol/CA | Flux (L·m−2·h−1·bar−1) | Bubble Point Pressure (MPa) | Maximum Pore Size (μm) | Porosity (%) | Viscosity (mPa·s) |
|---|---|---|---|---|---|---|
| 9 | 1.5 | 2383.6 ± 25.6 | 0.124 ± 0.002 | 2.33 | 87.5 ± 2.1 | 947.5 |
| 10 | 2.0 | 4013.4 ± 120.2 | 0.265 ± 0.001 | 1.09 | 88.8 ± 2.0 | 1062.0 |
| 11 | 2.5 | 10,063.6 ± 320.1 | 0.450 ± 0.005 | 0.64 | 91.4 ± 1.5 | 1174.8 |
| 12 | 3.0 | 24,472.7 ± 550.5 | 0.470 ± 0.010 | 0.61 | 93.2 ± 1.8 | 1207.5 |
| NO | Vaporization Temperature (°C) | Flux (L·m−2·h−1·bar−1) | Bubble Point Pressure (MPa) | Maximum Pore Size (μm) |
|---|---|---|---|---|
| a | 25 | 5681.9 ± 230.1 | 0.150 ± 0.002 | 1.92 |
| b | 55 | 3386.1 ± 160.5 | 0.160 ± 0.007 | 1.80 |
| c | 100 | 3036.4 ± 75.9 | 0.245 ± 0.010 | 1.18 |
| d | 150 | 2602.7 ± 60.6 | 0.355 ± 0.015 | 0.81 |
| NO | Vaporization Time (S) | Flux (L·m−2·h−1·bar−1) | Bubble Point Pressure (MPa) | Maximum Pore Size (μm) |
|---|---|---|---|---|
| e | 10 | 6394.4 ± 235.1 | 0.170 ± 0.006 | 1.70 |
| f | 30 | 6341.7 ± 205.2 | 0.240 ± 0.005 | 1.20 |
| g | 60 | 3366.6 ± 78.2 | 0.260 ± 0.008 | 1.11 |
| h | 120 | 2196.6 ± 50.6 | 0.280 ± 0.012 | 1.03 |
| NO | Humidity (%) | Flux (L·m−2·h−1·bar−1) | Bubble Point Pressure (MPa) | Maximum Pore Size (μm) |
|---|---|---|---|---|
| i | 30 | 4423.2 ± 178.2 | 0.110 ± 0.002 | 2.62 |
| j | 60 | 10,248.3 ± 325.2 | 0.145 ± 0.005 | 1.99 |
| k | ≥90 | 12,322.7 ± 480.5 | 0.285 ± 0.012 | 1.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Song, H.; Ren, L.; Talukder, M.E.; Chen, S.; Shao, J. Study on the Preparation of Cellulose Acetate Separation Membrane and New Adjusting Method of Pore Size. Membranes 2022, 12, 9. https://doi.org/10.3390/membranes12010009
Wang J, Song H, Ren L, Talukder ME, Chen S, Shao J. Study on the Preparation of Cellulose Acetate Separation Membrane and New Adjusting Method of Pore Size. Membranes. 2022; 12(1):9. https://doi.org/10.3390/membranes12010009
Chicago/Turabian StyleWang, Jianming, Hongchen Song, Longfei Ren, Md Eman Talukder, Shunquan Chen, and Jiahui Shao. 2022. "Study on the Preparation of Cellulose Acetate Separation Membrane and New Adjusting Method of Pore Size" Membranes 12, no. 1: 9. https://doi.org/10.3390/membranes12010009