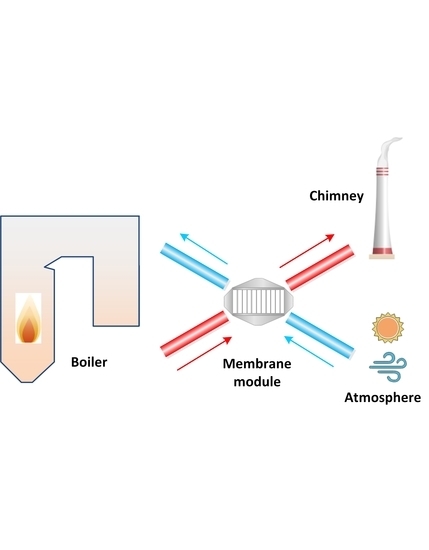
Experimental Study on a Ceramic Membrane Condenser with Air Medium for Water and Waste Heat Recovery from Flue Gas
Abstract
:1. Introduction
2. Method and Experiments System
2.1. Membrane Materials
2.2. System Overview
- (1)
- When valves 2, 4 are opened, and 1, 3 are closed, the wet flue gas at the outlet of the flue gas generation subsystem directly enters the flue gas drying subsystem. The flue gas drying subsystem measures the volume of condensed water in the water collection bottle II and the weight gain of the drying tower under different flue gas flow, and then determines the supersaturation coefficient of the wet flue gas in the experimental system.
- (2)
- When valves 1, 3 are opened and 2, 4 are closed, the wet flue gas at the outlet of the flue gas generation subsystem enters the shell side of the ceramic membrane module, and a series of flue gas water and waste heat recovery experiments are carried out.
2.3. Heat and Mass Transport Model
2.4. Methods
2.4.1. Supersaturation Coefficient
2.4.2. Water Recovery Characteristics
2.4.3. Heat Recovery Characteristics
2.4.4. Moisture Characteristics of Negative Pressure Air
2.4.5. Sensitivity Analysis
3. Results and Discuss
3.1. Supersaturation Coefficient of Flue Gas
3.2. Water and Heat Recovery Characteristics
3.2.1. Effect of Flue Gas Flow
3.2.2. Effect of Flue Gas Temperature
3.2.3. Effect of Negative Pressure Airflow
3.2.4. Effect of Membrane Pore Size
3.2.5. The Driving Force for Water and Waste Heat Recovery
3.3. Sensitivity Analysis
3.4. Latent/Sensible Heat Recovery Characteristics
3.5. Negative Pressure Air Characteristics
4. Conclusions
- (1)
- The flue gas temperature is the most sensitive factor related to the water and waste heat recovery characteristics from the flue gas. Increasing the flue gas temperature helps to increase the water recovery rate and heat recovery power from flue gas, but the water recovery efficiency and heat recovery efficiency will increase first and then decrease. Increasing the flue gas flow can increase the heat recovery power, but it will not continue to increase the water recovery rate. At the same time, it will reduce the water recovery efficiency and heat recovery efficiency. When the negative gas airflow is maximum or zero, the water and heat recovery performance are better, but the high vacuum degree has the risk of infiltration of non-condensable gas. Except for the 0.4 nm ceramic membrane, the membranes with different pore sizes have little effect on the water and waste heat recovery characteristics from flue gas.
- (2)
- The waste heat recovery of flue gas is dominated by latent heat recovery of water vapor, accounting for 80% and above. The negative pressure air is heated and humidified by the ceramic membrane condenser, and the negative pressure air outlet temperature has a significant promote. Compared with the high vacuum, increasing the negative pressure airflow has more practical application possibilities. Thus, a pore size of 0.4 nm in ceramic membranes is too small, and is not suitable for water and waste heat recovery from flue gas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IEA. World Energy Balances: Overview, IEA, Paris. 2021. Available online: https://www.iea.org/reports/world-energy-balances-overview (accessed on 13 September 2021).
- Chen, H.; Zhou, Y.; Su, X.; Cao, S.; An, L. Experimental study of water recovery from flue gas using hollow micro-nano porous ceramic composite membranes. J. Ind. Eng. Chem. 2018, 57, 349–355. [Google Scholar] [CrossRef]
- Wang, D. Transport Membrane Condenser for Water and Energy Recovery from Power Plant Flue Gas; Gas Technology Institute: Des Plaines, IL, USA, 2012. [Google Scholar]
- Wang, D.; Bao, A.; Kunc, W.; Liss, W. Coal power plant flue gas waste heat and water recovery. Appl. Energy 2012, 91, 341–348. [Google Scholar] [CrossRef]
- Bao, A.; Wang, D.; Lin, C. Nanoporous membrane tube condensing heat transfer enhancement study. Int. J. Heat Mass Transf. 2015, 84, 456–462. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Chen, H.; Cheng, C.; Liang, K. Moisture and latent heat recovery from flue gas by nonporous organic membranes. J. Clean. Prod. 2019, 225, 1065–1078. [Google Scholar] [CrossRef]
- Drioli, E.; Santoro, S.; Simone, S.; Barbieri, G.; Brunetti, A.; Macedonio, F.; Figoli, A. ECTFE membrane preparation for recovery of humidified gas streams using membrane condenser. React. Funct. Polym. 2014, 79, 1–7. [Google Scholar] [CrossRef]
- Jia, L.; Peng, X.F.; Sun, J.D.; Chen, T.B. An experimental study on vapor condensation of wet flue gas in a plastic heat exchange. Heat Transf. Asian Res. 2010, 30, 571–580. [Google Scholar] [CrossRef]
- Wang, L.; He, Y.; Tang, C.; Che, D. A novel design of rotary regenerative condensing heat exchanger for the dehydration from high humidity flue gas. Int. J. Heat Mass Transf. 2019, 131, 517–526. [Google Scholar] [CrossRef]
- Jeong, K.; Kessen, M.J.; Bilirgen, H.; Levy, E.K. Analytical modelling of water condensation in condensing heat exchanger. Int. J. Heat Mass Transf. 2010, 53, 2361–2368. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, Y.; Fu, L.; Zhang, S. Experimental and theoretical investigation of a novel full-open absorption heat pump applied to district heating by recovering waste heat of flue gas. Energy Build. 2018, 173, 45–57. [Google Scholar] [CrossRef]
- Aly, A.A.; Zeidan, B.E.; Hamed, A.M. Solar-powered open absorption cycle modeling with two desiccant solutions. Energy Convers. Manag. 2011, 52, 2768–2776. [Google Scholar] [CrossRef]
- Wei, M.; Yuan, W.; Song, Z.; Fu, L.; Zhang, S. Simulation of a heat pump system for total heat recovery from flue gas. Appl. Therm. Eng. 2015, 86, 326–332. [Google Scholar] [CrossRef]
- Brunetti, A.; Santoro, S.; Macedonio, F.; Figoli, A.; Drioli, E.; Barbieri, G. Waste gaseous streams: From environmental issue to source of water by using membrane condensers. CLEAN Soil Air Water 2014, 42, 1145–1153. [Google Scholar] [CrossRef]
- Fakharnezhad, A.; Keshavarz, P. Experimental investigation of gas dehumidification by tri-ethylene glycol in hollow fiber membrane contactors. J. Ind. Eng. Chem. 2016, 34, 390–396. [Google Scholar] [CrossRef]
- Wang, J.; Hua, J.; Fu, L.; Wang, Z.; Zhang, S. A theoretical fundamental investigation on boilers equipped with vapor-pump system for flue-gas heat and moisture recovery. Energy 2019, 171, 956–970. [Google Scholar] [CrossRef]
- Kuck, J. Efficiency of vapor pump equipped condensing boilers. Appl. Therm. Eng. 1996, 16, 233–244. [Google Scholar] [CrossRef]
- Wang, T.; Yue, M.; Qi, H.; Feron, P.H.M.; Zhao, S. Transport membrane condenser for water and heat recovery from gaseous streams: Performance evaluation. J. Membr. Sci. 2015, 484, 10–17. [Google Scholar] [CrossRef]
- Zhao, S.; Feron, P.H.M.; Xie, Z.; Zhang, J.; Hoang, M. Condensation studies in membrane evaporation and sweeping gas membrane distillation. J. Memb. Sci. 2014, 462, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Novak, P.J.; Hozalski, R.M.; Cussler, E.L.; Semmens, M.J. Condensation studies in gas permeable membranes. J. Membr. Sci. 2004, 231, 47–55. [Google Scholar] [CrossRef]
- Wang, D. Advanced Energy and Water Recovery Technology from Low Grade Waste Heat; Office of Scientific & Technical Information Technical Reports: Des Plaines, IL, USA, 2011. [Google Scholar]
- Zhou, Y.; Chen, H.; Xie, T.; Wang, B.; An, L. Effect of mass transfer on heat transfer of microporous ceramic membranes for water recovery. Int. J. Heat Mass Transf. 2017, 112, 643–648. [Google Scholar] [CrossRef]
- Hu, H.; Tang, G.; Niu, D. Wettability modified nanoporous ceramic membrane for simultaneous residual heat and condensate recovery. Sci. Rep. 2016, 6, 27274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, Z.; Zhang, H.; Chen, H.; Gao, D. Numerical study on recovering moisture and heat from flue gas by means of a macroporous ceramic membrane module. Energy 2020, 207, 118230. [Google Scholar] [CrossRef]
- Li, Z.; Xue, K.; Zhang, H.; Chen, H.; Gao, D. Numerical investigation on condensation mode of the transport membrane condenser. Int. J. Heat Mass Transf. 2020, 161, 120305. [Google Scholar] [CrossRef]
- Cheng, C.; Fu, H.; Zhang, H.; Chen, H.; Gao, D. Study on the preparation and properties of talcum-fly ash based ceramic membrane supports. Membranes 2020, 10, 207. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Cao, S.; Li, X.; Su, X.; An, L.; Gao, D. Heat exchange and water recovery experiments of flue gas with using nanoporous ceramic membranes. Appl. Therm. Eng. 2017, 110, 686–694. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Sun, J.; Liu, Y.; Zhong, Y.; Du, W.; Lan, J. An experimental study of membranes for capturing water vapor from flue gas. J. Energy Inst. 2018, 91, 339–348. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Yang, X.; Ru, K.; Song, J. Simulation of water recovery in membrane condenser dehumidification process. Appl. Therm. Eng. 2021, 193, 117018. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, M.; Yuan, W.; Huang, S. Microporous ceramic membrane condenser for water and heat recovery from flue gas. Appl. Therm. Eng. 2021, 186, 116512. [Google Scholar] [CrossRef]
- Lee, H.K.; Hwang, S.T. The transport of condensable vapours through a microporous voter glass membrane. J. Colloid Interface Sci. 1986, 110, 544–555. [Google Scholar] [CrossRef]
- Yue, M.; Zhao, S.; Feron, P.H.M.; Qi, H. Multichannel tubular ceramic membrane for water and heat recovery from waste gas streams. Ind. Eng. Chem. Res. 2016, 55, 2615–2622. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H. Heat and water recovery from flue gas: Application of microporous ceramic membrane tube bundles in gas-fired power plant. Chem. Eng. Process. Process. Intensif. 2019, 137, 116–127. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, H.; Chen, H. Experimental study on water recovery from flue gas using microporous ceramic membranes. Materials 2020, 13, 804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Li, X.; Wei, J.; Feng, Y.; Gao, D. Preparation and properties of coal ash ceramic membranes for water and heat recovery from flue gas. J. Chem. 2019, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Li, Z.; Zhang, H.; Zhang, J.; Chen, H.; Fu, H. Moisture recovery from gas-fired boiler exhaust using membrane module array. J. Clean. Prod. 2019, 231, 1110–1121. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Chen, H.; Zhang, J.; Chen, C. Experimental research on the heat transfer and water recovery performance of transport membrane condenser. Appl. Therm. Eng. 2019, 160, 114060. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Li, Z. Evaluation of a flue gas driven open absorption system for heat and water recovery from fossil fuel boilers. Energy Convers. Manag. 2016, 128, 57–65. [Google Scholar] [CrossRef]
- Soleimanikutanaei, S.; Lin, C.X.; Wang, D. Numerical modelling and analysis of transport membrane condensers for waste heat and water recovery from flue gas. Int. J. Therm. Sci. 2019, 136, 96–106. [Google Scholar] [CrossRef]



























| Project | Unit | Ceramic Membrane | Shell | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Average pore size | nm | 0.4 | 10 | 100 | 1000 | / |
| Structure | / | Asymmetric | Symmetry | |||
| Coating | / | Outer coating | / | / | ||
| Material | / | Alumina | AISI 316L | |||
| Length | mm | 790 | 790 | 790 | 790 | 800 |
| Outer diameter | mm | 12 | 22 | |||
| Inter diameter | mm | 8 | 20 | |||
| Porosity | % | 31.56% | 33.62% | 33.86% | 34.12% | / |
| Outer surface area | cm2 | 297.67 | / | |||
| Inner surface area | cm2 | 198.45 | / | |||
| Flow area | cm2 | 0.50 | 2.01 | |||
| Parameter | Unit | Value | Parameter | Unit | Value |
|---|---|---|---|---|---|
| Flue gas temperature | °C | 38–64 | Negative pressure air temperature | °C | 12–14 |
| Flue gas flow | L/min | 4–22 | Negative pressure airflow | L/h | 0–15 |
| Instrument | Model | Range | Uncertainty |
|---|---|---|---|
| Metal rotor flowmeter | CGYL-LZ-25 | 0–30 L/min | 1.0% |
| Glass rotor flowmeter | LWGY-4-C-10 | 0–20 L/min | 1.0% |
| Temperature transmitter | SWB-B | 0–100 °C | 0.25% |
| Pressure transmitter | CGYL-202 | −50–50 kPa | 0.25% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, D.; An, L.; Shen, G.; Zhang, S.; Zhang, H. Experimental Study on a Ceramic Membrane Condenser with Air Medium for Water and Waste Heat Recovery from Flue Gas. Membranes 2021, 11, 701. https://doi.org/10.3390/membranes11090701
Teng D, An L, Shen G, Zhang S, Zhang H. Experimental Study on a Ceramic Membrane Condenser with Air Medium for Water and Waste Heat Recovery from Flue Gas. Membranes. 2021; 11(9):701. https://doi.org/10.3390/membranes11090701
Chicago/Turabian StyleTeng, Da, Liansuo An, Guoqing Shen, Shiping Zhang, and Heng Zhang. 2021. "Experimental Study on a Ceramic Membrane Condenser with Air Medium for Water and Waste Heat Recovery from Flue Gas" Membranes 11, no. 9: 701. https://doi.org/10.3390/membranes11090701