Proton Conductive, Low Methanol Crossover Cellulose-Based Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sulfated Cellulose Solution
2.3. Fabrication of Crosslinked Sulfated Cellulose Electrolyte Membranes
2.4. Membrane Characterization
2.4.1. Characterization of Morphology and Structure
2.4.2. Thermal Stability
2.4.3. Mechanical Stability
2.4.4. Contact Angle and Water/Methanol Uptake
2.4.5. Ionic Conductivity
2.4.6. Methanol Permeability
3. Results
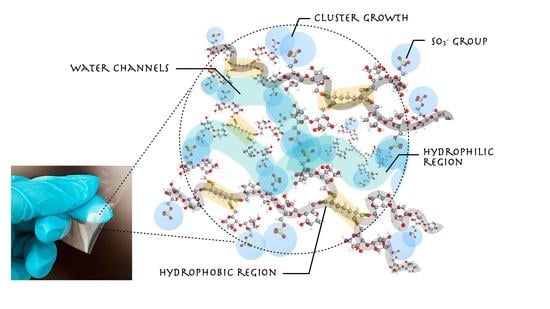
3.1. Crosslinked Membrane Structure and Morphology Characterization:
3.2. Thermal/Mechanical Stability and Contact Angle Measurement
3.3. Water/Methanol Uptake, Proton Conductivity and Methanol Crossover
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shafiee, S.; Topal, E. An econometrics view of worldwide fossil fuel consumption and the role of US. Energy Policy 2008, 36, 775–786. [Google Scholar] [CrossRef]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Hoogers, G. Fuel Cell Technology Handbook, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef] [Green Version]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahman, S.M.H.; Eriksson, S.-G.; Azad, A.K. Proton-conducting electrolytes for direct methanol and direct urea fuel cells—A state-of-the-art review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Kamaruddin, M.Z.F.; Kamarudin, S.K.; Daud, W.R.W.; Masdar, M.S. An overview of fuel management in direct methanol fuel cells. Renew. Sustain. Energy Rev. 2013, 24, 557–565. [Google Scholar] [CrossRef]
- Akinyele, D.; Olabode, E.; Amole, A. Review of Fuel Cell Technologies and Applications for Sustainable Microgrid Systems. Inventions 2020, 5, 42. [Google Scholar] [CrossRef]
- Yao, B.; Yan, X.; Ding, Y.; Lu, Z.; Dong, D.; Ishida, H.; Litt, M.; Zhu, L. Synthesis of Sulfonic Acid-Containing Polybenzoxazine for Proton Exchange Membrane in Direct Methanol Fuel Cells. Macromolecules 2014, 47, 1039–1045. [Google Scholar] [CrossRef]
- Li, J.; Cai, W.; Ma, L.; Zhang, Y.; Chen, Z.; Cheng, H. Towards neat methanol operation of direct methanol fuel cells: A novel self-assembled proton exchange membrane. Chem. Commun. 2015, 51, 6556–6559. [Google Scholar] [CrossRef]
- Ru, C.; Li, Z.; Zhao, C.; Duan, Y.; Zhuang, Z.; Bu, F.; Na, H. Enhanced Proton Conductivity of Sulfonated Hybrid Poly(arylene ether ketone) Membranes by Incorporating an Amino–Sulfo Bifunctionalized Metal–Organic Framework for Direct Methanol Fuel Cells. ACS Appl. Mater. Interfaces 2018, 10, 7963–7973. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Dimitrova, P.; Friedrich, K.A.; Stimming, U.; Vogt, B. Modified Nafion®-based membranes for use in direct methanol fuel cells. Solid State Ion. 2002, 150, 115–122. [Google Scholar] [CrossRef]
- Rhee, C.H.; Kim, H.K.; Chang, H.; Lee, J.S. Nafion/Sulfonated Montmorillonite Composite: A New Concept Electrolyte Membrane for Direct Methanol Fuel Cells. Chem. Mater. 2005, 17, 1691–1697. [Google Scholar] [CrossRef]
- Chien, H.-C.; Tsai, L.-D.; Huang, C.-P.; Kang, C.-y.; Lin, J.-N.; Chang, F.-C. Sulfonated graphene oxide/Nafion composite membranes for high-performance direct methanol fuel cells. Int. J. Hydrog. Energy 2013, 38, 13792–13801. [Google Scholar] [CrossRef]
- Li, J.; Bu, F.; Ru, C.; Jiang, H.; Duan, Y.; Sun, Y.; Pu, X.; Shang, L.; Li, X.; Zhao, C. Enhancing the selectivity of Nafion membrane by incorporating a novel functional skeleton molecule to improve the performance of direct methanol fuel cells. J. Mater. Chem. A 2020, 8, 196–206. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Liu, C.; Li, Y.; Xing, W.; Sun, J. Self-Healing Proton-Exchange Membranes Composed of Nafion–Poly(vinyl alcohol) Complexes for Durable Direct Methanol Fuel Cells. Adv. Mater. 2018, 30, 1707146. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrog. Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Jia, W.; Tang, B.; Wu, P. Novel Composite Proton Exchange Membrane with Connected Long-Range Ionic Nanochannels Constructed via Exfoliated Nafion–Boron Nitride Nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 14791–14800. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, C.; Lu, T.; Xing, W. Polyelectrolyte complexes of chitosan and phosphotungstic acid as proton-conducting membranes for direct methanol fuel cells. J. Power Sources 2007, 167, 94–99. [Google Scholar] [CrossRef]
- Choi, B.G.; Hong, J.; Park, Y.C.; Jung, D.H.; Hong, W.H.; Hammond, P.T.; Park, H. Innovative Polymer Nanocomposite Electrolytes: Nanoscale Manipulation of Ion Channels by Functionalized Graphenes. ACS Nano 2011, 5, 5167–5174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, Z.; Liu, C.; Xing, W.; Zhang, J. Implantation of Nafion® ionomer into polyvinyl alcohol/chitosan composites to form novel proton-conducting membranes for direct methanol fuel cells. J. Power Sources 2009, 194, 730–736. [Google Scholar] [CrossRef]
- Walkowiak-Kulikowska, J.; Wolska, J.; Koroniak, H. Chapter 18—Biopolymer membranes in fuel cell applications. In Biopolymer Membranes and Films; Elsevier: Amsterdam, The Netherlands, 2020; pp. 423–476. [Google Scholar]
- Miyamoto, H.; Sakakibara, K.; Wataoka, I.; Tsujii, Y.; Yamane, C.; Kajiwara, K. Interaction of Water Molecules with Carboxyalkyl Cellulose. In Cellulose Science and Technology: Chemistry, Analysis, and Applications; Wiley: Hoboken, NJ, USA, 2018; pp. 127–141. [Google Scholar]
- Ghassemi, Z.; Slaughter, G. Biological Fuel Cells and Membranes. Membranes 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Morais, J.D.; Silva, A.C.Q.; Muñoz-Gil, D.; Figueiredo, F.M.L.; Silvestre, A.J.D.; Freire, C.S.R. Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells. Nanomaterials 2020, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Selyanchyn, O.; Selyanchyn, R.; Lyth, S.M. A Review of Proton Conductivity in Cellulosic Materials. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Sriruangrungkamol, A.; Chonkaew, W. Modification of nanocellulose membrane by impregnation method with sulfosuccinic acid for direct methanol fuel cell applications. Polym. Bull. 2021, 78, 3705–3728. [Google Scholar] [CrossRef]
- Seo, J.A.; Kim, J.C.; Koh, J.K.; Ahn, S.H.; Kim, J.H. Preparation and characterization of crosslinked cellulose/sulfosuccinic acid membranes as proton conducting electrolytes. Ionics 2009, 15, 555–560. [Google Scholar] [CrossRef]
- Yang, J.; Sun, D.; Li, J.; Yang, X.; Yu, J.; Hao, Q.; Liu, W.; Liu, J.; Zou, Z.; Gu, J. In situ deposition of platinum nanoparticles on bacterial cellulose membranes and evaluation of PEM fuel cell performance. Electrochim. Acta 2009, 54, 6300–6305. [Google Scholar] [CrossRef]
- Guccini, V.; Carlson, A.; Yu, S.; Lindbergh, G.; Lindström, R.W.; Salazar-Alvarez, G. Highly proton conductive membranes based on carboxylated cellulose nanofibres and their performance in proton exchange membrane fuel cells. J. Mater. Chem. A 2019, 7, 25032–25039. [Google Scholar] [CrossRef] [Green Version]
- Budtova, T.; Navard, P. Cellulose in NaOH–water based solvents: A review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-L.; Zhang, X.; You, T.-T.; Xu, F. Deep eutectic solvents (DESs) for cellulose dissolution: A mini-review. Cellulose 2019, 26, 205–213. [Google Scholar] [CrossRef]
- Kim, D.; Livazovic, S.; Falca, G.; Nunes, S.P. Oil–Water Separation using Membranes Manufactured from Cellulose/Ionic Liquid Solutions. ACS Sustain. Chem. Eng. 2019, 7, 5649–5659. [Google Scholar] [CrossRef] [Green Version]
- Puspasari, T.; Yu, H.; Peinemann, K.-V. Charge- and Size-Selective Molecular Separation using Ultrathin Cellulose Membranes. ChemSusChem 2016, 9, 2908–2911. [Google Scholar] [CrossRef]
- Hu, T.Q.; Hashaikeh, R.; Berry, R.M. Isolation of a novel, crystalline cellulose material from the spent liquor of cellulose nanocrystals (CNCs). Cellulose 2014, 21, 3217–3229. [Google Scholar] [CrossRef]
- Zhang, K.; Brendler, E.; Geissler, A.; Fischer, S. Synthesis and spectroscopic analysis of cellulose sulfates with regulable total degrees of substitution and sulfation patterns via 13C NMR and FT Raman spectroscopy. Polymer 2011, 52, 26–32. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Ukkola, J.; Liimatainen, H. Direct sulfation of cellulose fibers using a reactive deep eutectic solvent to produce highly charged cellulose nanofibers. Cellulose 2019, 26, 2303–2316. [Google Scholar] [CrossRef] [Green Version]
- Shao, L.-L.; An, Q.-F.; Ji, Y.-L.; Zhao, Q.; Wang, X.-S.; Zhu, B.-K.; Gao, C.-J. Preparation and characterization of sulfated carboxymethyl cellulose nanofiltration membranes with improved water permeability. Desalination 2014, 338, 74–83. [Google Scholar] [CrossRef]
- Lalia, B.S.; Alkaabi, M.; Hashaikeh, R. Sulfated Cellulose/Polyvinyl Alcohol Composites as Proton Conducting Electrolyte for Capacitors. Energy Procedia 2015, 75, 1869–1874. [Google Scholar] [CrossRef] [Green Version]
- Ciolacu, D.E.; Suflet, D.M. 11-Cellulose-Based Hydrogels for Medical/Pharmaceutical Applications. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Popa, V., Volf, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–439. [Google Scholar]
- Puspasari, T.; Pradeep, N.; Peinemann, K.-V. Crosslinked cellulose thin film composite nanofiltration membranes with zero salt rejection. J. Membr. Sci. 2015, 491, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Jeon, Y.; Kim, D.; Kwon, G.; Kim, U.-J.; Hong, C.; Choung, J.W.; You, J. Double-crosslinked cellulose nanofiber based bioplastic films for practical applications. Carbohydr. Polym. 2021, 260, 117817. [Google Scholar] [CrossRef]
- Wang, M.; Jia, X.; Liu, W.; Lin, X. Water insoluble and flexible transparent film based on carboxymethyl cellulose. Carbohydr. Polym. 2021, 255, 117353. [Google Scholar] [CrossRef]
- Zhao, G.; Lyu, X.; Lee, J.; Cui, X.; Chen, W.-N. Biodegradable and transparent cellulose film prepared eco-friendly from durian rind for packaging application. Food Packag. Shelf Life 2019, 21, 100345. [Google Scholar] [CrossRef]
- Xu, G.G.; Yang, C.Q.; Deng, Y. Combination of bifunctional aldehydes and poly(vinyl alcohol) as the crosslinking systems to improve paper wet strength. J. Appl. Polym. Sci. 2004, 93, 1673–1680. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Rojas, J.; Azevedo, E. Functionalization and crosslinking of microcrystalline cellulose in aqueous media: A safe and economic approach. Int. J. Pharm. Sci. Rev. Res. 2011, 8, 28–36. [Google Scholar]
- Lalia, B.S.; Samad, Y.A.; Hashaikeh, R. Nanocrystalline-cellulose-reinforced poly(vinylidenefluoride-co-hexafluoropropylene) nanocomposite films as a separator for lithium ion batteries. J. Appl. Polym. Sci. 2012, 126, E442–E448. [Google Scholar] [CrossRef]
- Chen, Z.; Holmberg, B.; Li, W.; Wang, X.; Deng, W.; Munoz, R.; Yan, Y. Nafion/Zeolite Nanocomposite Membrane by in Situ Crystallization for a Direct Methanol Fuel Cell. Chem. Mater. 2006, 18, 5669–5675. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, B.; Zhao, J.; Chen, H. Improved process for the production of cellulose sulfate using sulfuric acid/ethanol solution. Carbohydr. Polym. 2013, 95, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on native cellulose and microcrystalline cellulose I structure studied by X-ray diffraction (WAXD): Comparison between measurement techniques. Lenzing. Ber. 2011, 89, 118–131. [Google Scholar]
- Zhang, H.; Xu, Y.; Li, Y.; Lu, Z.; Cao, S.; Fan, M.; Huang, L.; Chen, L. Facile Cellulose Dissolution and Characterization in the Newly Synthesized 1,3-Diallyl-2-ethylimidazolium Acetate Ionic Liquid. Polymers 2017, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Lee, Y.; Pettersson, P.O.; Torget, R.W. Heterogeneous aspects of acid hydrolysis of α-cellulose. In Biotechnology for Fuels and Chemicals; Springer: Berlin/Heidelberg, Germany, 2003; pp. 505–514. [Google Scholar]
- Hou, T.; Guo, K.; Wang, Z.; Zhang, X.-F.; Feng, Y.; He, M.; Yao, J. Glutaraldehyde and polyvinyl alcohol crosslinked cellulose membranes for efficient methyl orange and Congo red removal. Cellulose 2019, 26, 5065–5074. [Google Scholar] [CrossRef]
- Hashaikeh, R.; Abushammala, H. Acid mediated networked cellulose: Preparation and characterization. Carbohydr. Polym. 2011, 83, 1088–1094. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of Sulfate Groups from Sulfuric Acid Hydrolysis on the Thermal Degradation Behavior of Bacterial Cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Staggs, J.E.J. Discrete bond-weighted random scission of linear polymers. Polymer 2006, 47, 897–906. [Google Scholar] [CrossRef]
- Jeon, J.G.; Kim, H.C.; Palem, R.R.; Kim, J.; Kang, T.J. Cross-linking of cellulose nanofiber films with glutaraldehyde for improved mechanical properties. Mater. Lett. 2019, 250, 99–102. [Google Scholar] [CrossRef]
- Lafitte, B.; Jannasch, P. Chapter Three—On the Prospects for Phosphonated Polymers as Proton-Exchange Fuel Cell Membranes. In Advances in Fuel Cells; Zhao, T.S., Kreuer, K.D., Van Nguyen, T., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2007; Volume 1, pp. 119–185. [Google Scholar]
- Kreuer, K.-D.; Rabenau, A.; Weppner, W. Vehicle Mechanism, A New Model for the Interpretation of the Conductivity of Fast Proton Conductors. Angew. Chem. Int. Ed. Engl. 1982, 21, 208–209. [Google Scholar] [CrossRef]
- Bergenstråhle, M.; Wohlert, J.; Himmel, M.E.; Brady, J.W. Simulation studies of the insolubility of cellulose. Carbohydr. Res. 2010, 345, 2060–2066. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Bellesia, G.; Chundawat, S.P.S.; Dale, B.E.; Langan, P.; Gnanakaran, S. Insights into Hydrogen Bonding and Stacking Interactions in Cellulose. J. Phys. Chem. A 2011, 115, 14191–14202. [Google Scholar] [CrossRef]
- Bao, Y.; Qian, H.-j.; Lu, Z.-y.; Cui, S. Revealing the Hydrophobicity of Natural Cellulose by Single-Molecule Experiments. Macromolecules 2015, 48, 3685–3690. [Google Scholar] [CrossRef]
- Medronho, B.; Duarte, H.; Alves, L.; Antunes, F.; Romano, A.; Lindman, B. Probing cellulose amphiphilicity. Nord. Pulp Pap. Res. J. 2015, 30, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Sponsler, O.L. Orientation of cellulose space lattice in the cell wall. Additional X-ray data from Valonia cell-wall. Protoplasma 1931, 12, 241–254. [Google Scholar] [CrossRef]
- Gierke, T.D.; Munn, G.E.; Wilson, F.C. The morphology in nafion perfluorinated membrane products, as determined by wide and small-angle x-ray studies. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687–1704. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Hugenschmidt, C.; Dickmann, M.; Abdel-Hady, E.E.; Mohamed, H.F.M.; Abdel-Hamed, M.O. Crosslinked PVA/SSA proton exchange membranes: Correlation between physiochemical properties and free volume determined by positron annihilation spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 28287–28299. [Google Scholar] [CrossRef]
- Zhang, H.; Stanis, R.J.; Song, Y.; Hu, W.; Cornelius, C.J.; Shi, Q.; Liu, B.; Guiver, M.D. Fuel cell performance of pendent methylphenyl sulfonated poly(ether ether ketone ketone)s. J. Power Sources 2017, 368, 30–37. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Selyanchyn, R.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. High Temperature Proton Conduction in Nanocellulose Membranes: Paper Fuel Cells. Chem. Mater. 2016, 28, 4805–4814. [Google Scholar] [CrossRef]
- Aricò, A.S.; Sebastian, D.; Schuster, M.; Bauer, B.; Urso, C.; Lufrano, F.; Baglio, V. Selectivity of Direct Methanol Fuel Cell Membranes. Membranes 2015, 5, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Simari, C.; Vecchio, C.L.; Enotiadis, A.; Davoli, M.; Baglio, V.; Nicotera, I. Toward optimization of a robust low-cost sulfonated-polyethersulfone containing layered double hydroxide for PEM fuel cells. J. Appl. Polym. Sci. 2019, 136, 47884. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aburabie, J.; Lalia, B.; Hashaikeh, R. Proton Conductive, Low Methanol Crossover Cellulose-Based Membranes. Membranes 2021, 11, 539. https://doi.org/10.3390/membranes11070539
Aburabie J, Lalia B, Hashaikeh R. Proton Conductive, Low Methanol Crossover Cellulose-Based Membranes. Membranes. 2021; 11(7):539. https://doi.org/10.3390/membranes11070539
Chicago/Turabian StyleAburabie, Jamaliah, Boor Lalia, and Raed Hashaikeh. 2021. "Proton Conductive, Low Methanol Crossover Cellulose-Based Membranes" Membranes 11, no. 7: 539. https://doi.org/10.3390/membranes11070539