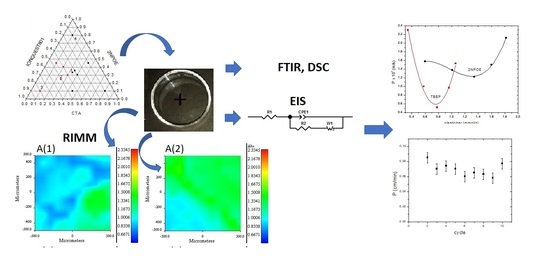
Structural Characterization of the Plasticizers’ Role in Polymer Inclusion Membranes Used for Indium (III) Transport Containing IONQUEST® 801 as Carrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
2.3. Synthesis of Polymeric Inclusion Membranes
2.4. Transport Experiments
2.5. In (III) Quantification
2.6. Liquid–Solid Extraction Experiments
2.7. Membrane Stability
2.8. EIS Characterization
2.9. RIMM Characterization
2.10. DSC Characterization
3. Results and Discussions
3.1. Evaluation of PIM Formation
3.2. PIM Thickness Characterization
3.3. Liquid–Solid Extraction Characterization
3.4. In (III) Permeability Profiles
3.5. FT-IR Characterization
3.6. RIMM Characterization
3.7. EIS Characterization
3.8. Thermal Analysis Characterization
3.9. PIM Stability Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953; ISBN 0801401348. [Google Scholar]
- Sears, J.K.; Darby, J.R. The Technology of Plasticizers; John Wiley & Sons: New York, NY, USA, 1982; p. 1174. ISBN 978-0471055839. [Google Scholar]
- Barshtein, R.S.; Kotlyarevskii, G.A. The mechanism of plasticization of poly (vinyl chloride). Plasticheskie Massy 1965, 7, 13–14. [Google Scholar]
- Barshtein, R.S.; Kotlyarevskii, G.A. Plasticizers for poly (vinyl chloride) and its copolymers. Plasticheskie Massy 1961, 2, 57–60. [Google Scholar]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P. Preparation and characterization of polymeric plasticized membranes (PPM) embedding a crown ether carrier application to copper ions transport. Mater. Sci. Eng. C 2005, 25, 436–443. [Google Scholar] [CrossRef]
- Kusumocahyo, S.P.; Kanamori, T.; Sumaru, K.; Aomatsu, S.; Matsuyama, H.; Teramoto, M.; Shinbo, T. Development of polymer inclusion membranes based on cellulose triacetate: Carrier–mediated transport of cerium (III). J. Membr. Sci. 2004, 244, 251–257. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Pathak, P.N.; Kelkar, A.; Manchanda, V.K. Novel polymer inclusion membrane containing macrocyclic ionophore for selective removal of strontium from nuclear waste solution. New J. Chem. 2004, 28, 1004–1009. [Google Scholar] [CrossRef]
- Sugiura, M. Effect of Polyoxyethylenen-Alkyl Ethers on Carrier-Mediated Transport of Lanthanide Ions through Cellulose Triacetate Membranes. Sep. Sci. Technol. 1992, 27, 269–276. [Google Scholar] [CrossRef]
- Fontas, C.; Tayeb, R.; Tingry, S.; Hidalgo, M.; Seta, P. Transport of platinum (IV) through supported liquid membrane (SLM) and polymeric plasticized membrane (PPM). J. Membr. Sci. 2005, 263, 96–102. [Google Scholar] [CrossRef]
- de Gyves, J.; Hernández-Andaluz, A.M.; Miguel, E.R.D.S. LIX®-loaded polymer inclusion membrane for copper(II) transport. J. Membr. Sci. 2006, 268, 142–149. [Google Scholar] [CrossRef]
- Miguel, E.R.D.S.; Aguilar, J.C.; de Gyves, J. Structural effects on metal ion migration across polymer inclusion membranes: Dependence of transport profiles on nature of active plasticizer. J. Membr. Sci. 2008, 307, 105–116. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W. Transport of Cr(VI), Zn(II), and Cd(II) Ions Across Polymer Inclusion Membranes with Tridecyl(pyridine) Oxide and Tri-n-Octylamine. Sep. Sci. Technol. 2004, 39, 3127–3141. [Google Scholar] [CrossRef]
- Scindia, Y.; Pandey, A.; Reddy, A. Coupled-diffusion transport of Cr(VI) across anion-exchange membranes prepared by physical and chemical immobilization methods. J. Membr. Sci. 2005, 249, 143–152. [Google Scholar] [CrossRef]
- Sugiura, M.; Kikkawa, M.; Urita, S. Effect of plasticizer on carrier–mediated transport of zinc ion through cellulose triacetate membranes. Sep. Sci. Technol. 1987, 22, 2263–2268. [Google Scholar] [CrossRef]
- Miguel, E.R.D.S.; Monroy-Barreto, M.; Aguilar, J.C.; Ocampo, A.L.; de Gyves, J. Structural effects on metal ion migration across polymer inclusion membranes: Dependence of membrane properties and transport profiles on the weight and volume fractions of the components. J. Membr. Sci. 2011, 379, 416–425. [Google Scholar] [CrossRef]
- Tarvainen, M.; Sutinen, R.; Somppi, M.; Paronen, P.; Poso, A. Predicting plasticization efficiency from three-dimensional molecular structure of a polymer plasticizer. Pharm. Res. 2001, 18, 1760–1766. [Google Scholar] [CrossRef]
- Paiva, A.P. Recovery of indium from aqueous solutions by solvent extraction. Sep. Sci. Technol. 2001, 36, 1395–1419. [Google Scholar] [CrossRef]
- Virolainen, S.; Ibana, D.; Paatero, E. Recovery of indium from indium tin oxide by solvent extraction. Hydrometall. 2011, 107, 56–61. [Google Scholar] [CrossRef]
- Meng, X.; Wang, C.; Zhou, P.; Xin, X.; Wang, L. Transport and selectivity of indium through polymer inclusion membrane in hydrochloric acid medium. Front. Environ. Sci. Eng. 2017, 11, 9. [Google Scholar] [CrossRef]
- Hayashita, T. Heavy Metal Ion Separation by Functional Polymeric Membranes. ACS Symposium Series 1996, 303–318. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.; Zoromba, M.S.; Abdel-Hamid, S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Monroy-Barreto, M.; Bautista-Flores, A.N.; Munguia-Acevedo, N.M.; Rodríguez de San Miguel, E.; de Gyves, J. Selective Palladium (II) Recovery Using a Polymer Inclusion Membrane with Tris(2-ethylhexyl) Phosphate (TEHP). Experimental and Theoretical Study. Ind. Eng. Chem. Res. 2021, 60, 3385–3396. [Google Scholar] [CrossRef]
- Liem, D.H.; Christian, S.D.; Grundnes, J.; Roach, S.R.; Engebretsen, J.E.; Ehrenberg, L. High-speed Computers as a Supplement to Graphical Methods. 12. Application of LETAGROP to Data for Liquid-liquid Distribution Equilibria. Acta Chem. Scand. 1971, 25, 1521–1534. [Google Scholar] [CrossRef]
- Fu, X.; Hu, Z.; Liu, Y.; Golding, J.A. Extraction of sodium in bis (2,44–trimethylpentyl) phosphinic acid CYANEX 272 TM: Basic constants and extraction equilibria. Solvent Extr. Ion Exch. 1990, 8, 573–575. [Google Scholar] [CrossRef]
- Rodriguez de San Miguel, E.; Garduño–Garcia, A.; Aguilar, J.C.; de Gyves, J. Gold (III) transport through polymer inclusion membranes: Efficiency factors and pertraction mechanism using Kelex 100 as carrier. Ind. Eng. Chem. Res. 2007, 46, 2861–2869. [Google Scholar] [CrossRef]
- Kebiche-Senhadji, O.; Mansouri, L.; Tingry, S.; Seta, P.; Benamor, M. Facilitated Cd(II) transport across CTA polymer inclusion membrane using anion (Aliquat 336) and cation (D2EHPA) metal carriers. J. Membr. Sci. 2008, 310, 438–445. [Google Scholar] [CrossRef]
- Arous, O. Comparison of carrier-facilitated silver (i) and copper (ii) ions transport mechanisms in a supported liquid membrane and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]
- Saji, J.; Reddy, M.L.P. Selective Extraction and Separation of Titanium (IV) from Multivalent Metal Chloride Solutions Using 2-Ethylhexyl Phosphonic Acid Mono-2-ethylhexyl Ester. Sep. Sci. Technol. 2003, 38, 427–444. [Google Scholar] [CrossRef]
- O’Rourke, M.; Duffy, N.; Marco, R.D.; Potter, I. Electrochemical Impedance Spectroscopy—A Simple Method for the Characterization of Polymer Inclusion Membranes Containing Aliquat 336. Membranes 2011, 1, 132–148. [Google Scholar] [CrossRef]










| PIM | CTA (mg) | Plasticizer (mg) | Ionquest® 801 (mg) | D | Log D |
|---|---|---|---|---|---|
| 11 | 30.0 | 10.5 (2NPOE) | 1.0 | 858.87 | 2.93 |
| 12 | 30.8 | 10.3 (2NPOE) | 1.4 | 5046.82 | 3.70 |
| 13 | 30.2 | 10.2 (2NPOE) | 1.7 | 11,638.95 | 4.07 |
| 14 | 30.3 | 10.2 (2NPOE) | 2.1 | 32,042.25 | 4.51 |
| 15 | 30.0 | 10.3 (2NPOE) | 2.5 | 31,892.52 | 4.50 |
| 16 | 30.5 | 10.1 (2NPOE) | 2.9 | 31,379.31 | 4.50 |
| 17 | 30.5 | 90.3 (2NPOE) | 1.0 | 80.33 | 1.90 |
| 18 | 30.1 | 90.1 (2NPOE) | 1.4 | 186.19 | 2.27 |
| 19 | 30.6 | 90.1 (2NPOE) | 1.9 | 411.42 | 2.61 |
| 20 | 30.5 | 90.0 (2NPOE) | 2.3 | 913.86 | 2.96 |
| 21 | 30.0 | 90.1 (2NPOE) | 2.6 | 1341.51 | 3.13 |
| 22 | 30.1 | 90.7 (2NPOE) | 2.9 | 1565.91 | 3.19 |
| 23 | 30.0 | 10.2 (TBEP) | 1.0 | 1224.26 | 3.09 |
| 24 | 30.8 | 9.9 (TBEP) | 1.3 | 2671.96 | 3.43 |
| 25 | 30.4 | 10.2 (TBEP) | 1.7 | 6987.13 | 3.84 |
| 26 | 30.0 | 10.6 (TBEP) | 2.2 | 31,892.52 | 4.50 |
| 27 | 30.6 | 10.6 (TBEP) | 2.6 | 31,164.38 | 4.49 |
| 28 | 30.6 | 10.2 (TBEP) | 3.1 | 31,093.39 | 4.49 |
| 29 | 30.8 | 90.4 (TBEP) | 0.9 | 205.01 | 2.31 |
| 30 | 30.8 | 90.2 (TBEP) | 1.5 | 483.12 | 2.68 |
| 31 | 29.7 | 90.3 (TBEP) | 1.8 | 786.28 | 2.89 |
| 32 | 30.3 | 90.1 (TBEP) | 2.2 | 1465.38 | 3.16 |
| 33 | 30.0 | 90.4 (TBEP) | 2.7 | 2400.94 | 3.38 |
| 34 | 30.2 | 90.3 (TBEP) | 3.1 | 2391.23 | 3.38 |
| Chemical Reaction | Equilibrium Constant |
|---|---|
| log β = 2.58 | |
| log β = 3.84 | |
| InCl3 | log β = 4.20 |
| log Kdim = 4.09 [27] |
| PIMs | Reaction | log K | U (σ) |
|---|---|---|---|
| 11–16 | 9.16 MAX 9.95 | 0.18 (0.14) | |
| 10.65 MAX 11.11 | |||
| 17–22 | 9.87 ± 0.13 | 0.05 (0.74) | |
| 23–28 | 8.59 MAX 9.17 | 0.13 (0.00) | |
| 10.55 MAX 10.87 | |||
| 29–34 | 9.47 ± 0.26 | 0.02 (0.03) | |
| 9.99 MAX 10.60 |
| PIM | CTA mg–(%w/w)–μmol | Plasticizer mg–(% w/w)–μmol | Ionquest(r) 801 mg–(% w/w)–μmol |
|---|---|---|---|
| 1 | 30.2–42.4 | 10.8–15.1–43.0 (2NPOE) | 30.3–42.5–98.9 |
| 2 | 30.6–37.7 | 20.5–25.2–81.6 (2NPOE) | 30.1–37.1–98.2 |
| 3 | 30.3–33.2 | 30.7–33.7–122.2 (2NPOE) | 30.2–33.1–98.5 |
| 4 | 30.3–30.0 | 40.4–40.0–160.7 (2NPOE) | 30.4–30.1–99.2 |
| 5 | 30.0–27.0 | 50.7–45.6–201.7 (2NPOE) | 30.5–27.4–99.5 |
| 6 | 30.7–42.6 | 10.6–14.7–25.0 (TBEP) | 30.8–42.7–100.5 |
| 7 | 30.8–37.5 | 20.5–25.0–48.3 (TBEP) | 30.8–37.5–100.5 |
| 8 | 30.5–33.3 | 30.4–33.2–71.7 (TBEP) | 30.7–33.5–100.2 |
| 9 | 30.3–29.9 | 40.7–40.2–96.0 (TBEP) | 30.3–29.9–98.9 |
| 10 | 30.8–27.5 | 50.6–45.2–119.4 (TBEP) | 30.4–27.2–99.2 |
| Compound | Band (cm−1) | Functional Group |
|---|---|---|
| CTA | 3600–3200 | O–H |
| CTA | 1735 | C=O |
| CTA | 1210–1035 | C–O–C |
| CTA | 2960–2850 | C–H |
| CTA | 1370 | C–H |
| 2NPOE | 1525 | NO2 |
| 2NPOE | 1351 | C–N |
| 2NPOE | 3480 | C–H (aromatic) |
| 2NPOE | 2960–2850 | –CH2– |
| 2NPOE | 720 | –CH2– |
| 2NPOE | 1235 | R–O–CH2 |
| 2NPOE | 1127 | C–O–C |
| 2NPOE | 1465 | –CH3 (octyl) |
| 2NPOE | 730–675 | C–H |
| Ionquest® 801 and TBEP | 1195 | P=O |
| Ionquest® 801 and TBEP | 1038 | P–OH (stretch) |
| Ionquest® 801 and TBEP | 1700–2700 | P–OH (dimeric form) |
| TBEP | 990 | P=O (TBEP) |
| PIM | CTA (mg) | Plasticizer (mg) | Ionquest® 801 (mg) | Mapped Frequency cm−1 |
|---|---|---|---|---|
| 39 | 30.8 | – | 30.8 | 801 |
| 40 | 30.7 | TBEP (30.6) | – | 1136 |
| 41 | 30.6 | 2NPOE (30.5) | – | 1528 |
| m2 | 30.7 | 2NPOE (20.2) | 30.9 | 1528 (2NPOE), 976 (Ionquest) |
| m4 | 30.3 | 2NPOE (40.2) | 30.2 | 1528 (2NPOE), 976 (Ionquest) |
| m7 | 30.2 | TBEP (20.7) | 30.2 | 1136 (TBEP) |
| m9 | 30.2 | TBEP (40.1) | 30.1 | 1136 (TEBP) |
| PIM | CTA (mg)–%w/w | Plasticizer (mg)–%w/w | Ionquest® 801 (mg)–%w/w | Thickness (µm) |
|---|---|---|---|---|
| M1 | (30.2)–80.11 | (3.7)–9.81 (2NPOE) | (3.8)–10.08 | 21.2 |
| M2 | (30.8)–45.36 | (6.8)–10.01 (2NPOE) | (30.3)–44.62 | 27.6 |
| M3 | (30.4)–33.44 | (30.5)–33.55 (2NPOE) | (30.0)–33.00 | 22.0 |
| M4 | (30.4)–45.24 | (30.2)–44.94 (2NPOE) | (6.6)–9.82 | 25.6 |
| M5 | (30.1)–79.84 | (3.9)–10.34 (TBEP) | (3.7)–9.81 | 19.2 |
| M6 | (30.2)–45.00 | (6.6)–9.84 (TBEP) | (30.3)–45.16 | 25.0 |
| M7 | (30.3)–33.30 | (30.5)–33.52 (TBEP) | (30.2)–33.19 | 31.2 |
| M8 | (30.6)–45.20 | (30.3)–44.76 (TBEP) | (6.8)–10.04 | 24.0 |
| PIM | R1 (Ω) | CPE1–T | CPE1–P | R2 (Ω) | W1–R | W1–T | W1–P | εr,m | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| PIMs without equilibration with the feed phase | |||||||||
| M1 | 58.25 | 3.21 × 10−9 | 1.004 | 92.27 | 62.64 | 0.0001904 | 0.44279 | 41.30 | 0.00023 |
| M2 | 54.18 | 4.53 × 10−9 | 0.974 | 85.03 | 43.10 | 0.0002498 | 0.40866 | 58.35 | 0.00015 |
| M3 | 61.22 | 2.04 × 10−9 | 1.008 | 99.92 | 43.63 | 0.0001819 | 0.42318 | 39.58 | 0.00019 |
| M4 | 53.70 | 2.85 × 10−9 | 1.011 | 94.02 | 37.92 | 0.0001469 | 0.41267 | 48.94 | 0.00016 |
| M5 | 57.15 | 4.55 × 10−9 | 0.988 | 71.93 | 29.45 | 0.0001832 | 0.38955 | 47.98 | 8.32 × 10−5 |
| M6 | 51.54 | 1.84 × 10−9 | 0.852 | 83.16 | 1.27 | 2.252 × 10−6 | 0.3644 | 54.03 | 0.00016 |
| M7 | 76.09 | 3.20 × 10−9 | 0.958 | 99.19 | 39.89 | 0.0002346 | 0.41140 | 56.64 | 0.00022 |
| M8 | 65.66 | 1.16 × 10−8 | 0.899 | 74.77 | 31.24 | 0.0002128 | 0.39015 | 57.70 | 0.00019 |
| PIMs in contact with a 0.1 mM In (III) solution for 5 min | |||||||||
| M1 | 50.28 | 3.07 × 10−9 | 0.992 | 78.08 | 31.15 | 0.0001350 | 0.41015 | 48.81 | 0.00026 |
| M2 | 60.50 | 3.81 × 10−9 | 0.985 | 61.25 | 41.96 | 0.0002229 | 0.42989 | 81.00 | 0.00017 |
| M3 | 65.07 | 2.55 × 10−9 | 0.984 | 69.84 | 28.91 | 0.0001204 | 0.40925 | 56.62 | 0.00039 |
| M4 | 57.63 | 2.45 × 10−9 | 1.025 | 64.20 | 35.74 | 0.0002531 | 0.43010 | 71.68 | 0.00015 |
| M5 | 54.72 | 5.11 × 10−9 | 0.994 | 69.06 | 38.08 | 0.0001678 | 0.36376 | 49.97 | 8.32 × 10−5 |
| M6 | 78.57 | 1.37 × 10−9 | 0.942 | 46.84 | 26.20 | 0.0001156 | 0.36756 | 95.94 | 0.00010 |
| M7 | 76.15 | 4.56 × 10−9 | 0.943 | 88.07 | 41.85 | 0.0002402 | 0.39235 | 63.68 | 0.00011 |
| M8 | 50.66 | 5.50 × 10−9 | 0.944 | 82.00 | 36.62 | 0.0001884 | 0.39304 | 52.61 | 8.66 × 10−5 |
| PIMs in contact with a 0.1 mM In (III) solution for 180 min | |||||||||
| M1 | 46.25 | 3.87 × 10−9 | 0.984 | 66.77 | 26.90 | 0.0002872 | 0.38189 | 57.07 | 0.00013 |
| M2 | 56.17 | 5.22 × 10−9 | 0.980 | 55.89 | 35.62 | 0.0002028 | 0.42254 | 88.77 | 9.73 × 10−5 |
| M3 | 60.70 | 2.57 × 10−9 | 0.992 | 64.86 | 30.56 | 0.0001717 | 0.39664 | 60.97 | 0.00019 |
| M4 | 56.73 | 2.59 × 10−9 | 1.020 | 57.44 | 33.92 | 0.0003145 | 0.38195 | 80.11 | 0.00015 |
| M5 | 52.99 | 2.24 × 10−6 | 0.988 | 68.35 | 45.22 | 0.0001791 | 0.40523 | 50.49 | 6.61 × 10−5 |
| M6 | 59.02 | 6.65 × 10−9 | 0.930 | 75.21 | 29.90 | 0.0002680 | 0.37830 | 59.75 | 0.00012 |
| M7 | 61.58 | 2.31 × 10−8 | 0.808 | 92.26 | 1.01 | 1.64 × 10−6 | 0.37316 | 60.79 | 0.00015 |
| M8 | 59.77 | 5.13 × 10−9 | 0.934 | 75.53 | 24.37 | 0.0001011 | 0.37324 | 57.12 | 0.00016 |
| PIM | CTA mg–mmol/g | Plasticizer mg–mmol/g | Ionquest® 801 mg–mmol/g | Tg (°C) | P × 105 m/s |
|---|---|---|---|---|---|
| 35 | 30.5 | 20.3–0.92 (2NPOE) | 35.7–1.35 | 262.99 | |
| 2 | 30.6 | 20.5–1.00 (2NPOE) | 30.1–1.21 | 1.36 | |
| 36 | 30.3 | 50.4–1.76 (2NPOE) | 32.2–0.93 | 230.99 | |
| 5 | 30.0 | 50.7–1.80 (2NPOE) | 30.5–0.89 | 2.11 | |
| 37 | 30.3 | 20.1–0.59 (TBEP) | 30.1–1.22 | 218.93 | |
| 7 | 30.8 | 20.5–0.59 (TBEP) | 30.8–1.22 | 1.01 | |
| 38 | 30.2 | 42.9–0.98 (TBEP) | 32.7–0.97 | 219.09 | |
| 9 | 30.3 | 40.7–0.95 (TBEP) | 30.3–0.98 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancilla-Rico, A.; de Gyves, J.; Rodríguez de San Miguel, E. Structural Characterization of the Plasticizers’ Role in Polymer Inclusion Membranes Used for Indium (III) Transport Containing IONQUEST® 801 as Carrier. Membranes 2021, 11, 401. https://doi.org/10.3390/membranes11060401
Mancilla-Rico A, de Gyves J, Rodríguez de San Miguel E. Structural Characterization of the Plasticizers’ Role in Polymer Inclusion Membranes Used for Indium (III) Transport Containing IONQUEST® 801 as Carrier. Membranes. 2021; 11(6):401. https://doi.org/10.3390/membranes11060401
Chicago/Turabian StyleMancilla-Rico, Alejandro, Josefina de Gyves, and Eduardo Rodríguez de San Miguel. 2021. "Structural Characterization of the Plasticizers’ Role in Polymer Inclusion Membranes Used for Indium (III) Transport Containing IONQUEST® 801 as Carrier" Membranes 11, no. 6: 401. https://doi.org/10.3390/membranes11060401