Permselectivity of Cation Exchange Membranes Modified by Polyaniline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objects of Research
2.2. Modification of Membranes by the PANI
2.3. Diffusion Permeability
2.4. Specific Conductivity
2.5. Current–Voltage Curves
2.6. Electrodialysis Treatment of Mixed Solutions
3. Results and Discussion
3.1. Influence of the PANI on Transport Properties of Ion Exchange Membranes
3.1.1. Diffusion Permeability
3.1.2. Specific Conductivity
3.1.3. Extended Tree-Wire Model
3.1.4. Current–Voltage Curves
3.1.5. Scanning Electron Microscopy
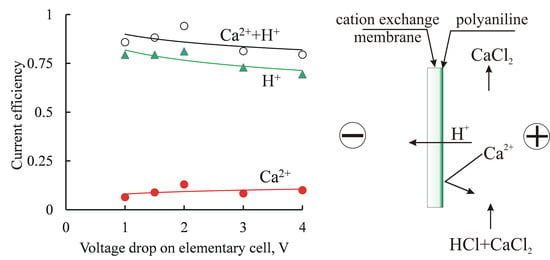
3.2. Electrodialysis Experiments
3.2.1. Heterogeneous Membranes
3.2.2. Homogeneous Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEM | Cation exchange membrane |
| CVC | Current–voltage curve |
| DP | Diffusion permeability |
| IEC | Ion exchange capacity |
| PANI | Polyaniline |
| a, b, c | Fractions of the current passing through the mixed, gel and solution channels |
| C | Concentration, mol-eq/L |
| ΔCi | Changes of i ion concentration during the experiment, mol-eq/m3 |
| d, e | Fractions of the solution and gel in the mixed channels |
| Di | Diffusion coefficient of i counter-ion in the solution, m2/s |
| F | Faraday constant, C/mol-eq |
| f1, f2 | Volume fractions of gel and solution pseudo-phases in membrane |
| Ji | Integral ion flux of i ions, mol-eq∙m−2∙s−1 |
| i | Current density, A/m2 |
| I | Current intensity, A |
| ilim | Limiting current density, A/m2 |
| K | Cell constant |
| Kd | Dimensionless conductivity of membrane gel phase |
| Km | Dimensionless conductivity of the membrane |
| l | Membrane thickness, m |
| n | Number of elementary cells in the electrodialysis unit |
| N | Number of CEMs in the electrodialysis unit |
| P | Diffusion permeability coefficient, m2/s |
| P1/2 | Permeability coefficient |
| Limiting permeability coefficient | |
| q | Solution volume velocity, m3/h |
| R | Resistance, Ohm |
| S | Membrane area, m2 |
| t | Time, s |
| t− | Transport number of co-ions in the solution |
| Counter-ion transport number in membrane | |
| U | Voltage applied on the electrodialysis unit, V |
| Uel.c. | Voltage applied on the elementary cell, V |
| V | Volume of the desalination chamber, m3 |
| Vw | Volume of the chamber with water, m3 |
| W | Energy consumption for the treatment of 1 m3 of the solution, W∙h/m3 |
| zA | Charge of co-ion |
| zi | Charge of i counter-ion |
| α | Structural parameter characterizing the spatial orientation of pseudo-phases inside membrane |
| ΔE | Potential drop, V |
| η | Current efficiency |
| κiso | Conductivity of membrane gel phase, S/m |
| κm | Conductivity of membrane, S/m |
| κsol | Conductivity of the solution, S/m |
References
- Li, C.; Ramasamy, D.L.; Sillanpää, M.; Repo, E. Separation and concentration of rare earth elements from wastewater using electrodialysis technology. Sep. Purif. Technol. 2021, 254, 117442. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis applications in wastewater treatment for environmental protection and resources recovery: A systematic review on progress and perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci. 2006, 119, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Afsar, N.U.; Gea, X.; Zhao, Z.; Hussain, A.; He, Y.; Ge, L.; Xu, T. Zwitterion membranes for selective cation separation via electrodialysis. Sep. Purif. Technol. 2021, 254, 117619. [Google Scholar] [CrossRef]
- Afsar, N.U.; Shehzad, M.A.; Irfan, M.; Emmanuel, K.; Sheng, F.; Xu, T.; Ren, X.; Ge, L.; Xu, T. Cation exchange membrane integrated with cationic and anionic layers for selective ion separation via electrodialysis. Desalination 2019, 458, 25–33. [Google Scholar] [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis—Effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Membr. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Sata, T.; Sata, T.; Yang, W. Studies on cation-exchange membranes having permselectivity between cations in electrodialysis. J. Membr. Sci. 2020, 206, 31–60. [Google Scholar] [CrossRef]
- Vaselbehagh, M.; Karkhanechi, H.; Takagi, R.; Matsuyama, H. Surface modification of an anion exchange membrane to improve the selectivity for monovalent anions in electrodialysis—Experimental verification of theoretical predictions. J. Membr. Sci. 2015, 490, 301–310. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Loza, N.V.; Loza, S.A.; Kononenko, N.A.; Magalyanov, A.V. Ion transport in sulfuric acid solution through anisotropic composites based on heterogeneous membranes and polyaniline. Petrol. Chem. 2015, 55, 724–729. [Google Scholar] [CrossRef]
- Blythe, T.; Bloor, D. Electrical Properties of Polymers, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005; p. 480. ISBN 0521552192. [Google Scholar]
- Farrokhzad, H.; Darvishmanesh, S.; Genduso, G.; Van Gerven, T.; Van der Bruggen, B. Development of bivalent cation selective ion exchange membranes by varying molecular weight of polyaniline. Electrochim. Acta 2015, 158, 64–72. [Google Scholar] [CrossRef]
- Kumar, M.; Khan, M.A.; AlOthman, Z.A.; Siddiqui, M.R. Polyaniline modified organic–inorganic hybrid cation-exchange membranes for the separation of monovalent and multivalent ions. Desalination 2013, 325, 95–103. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K.; Trivedi, G.S.; Rangarajan, R. Preparation and electrochemical characterization of cation- and anion-exchange/polyaniline composite membranes. J. Colloid Interface Sci. 2004, 277, 162–171. [Google Scholar] [CrossRef]
- Chamoulaud, G.; Belanger, D. Modification of ion-exchange membrane used for separation of protons and metallic cations and characterization of the membrane by current–voltage curves. J. Colloid Interface Sci. 2005, 281, 179–187. [Google Scholar] [CrossRef]
- Amado, F.D.R.; Rodrigues, M.A.S.; Morisso, F.D.P.; Bernardes, A.M.; Ferreira, J.Z.; Ferreira, C.A. High-impact polystyrene/polyaniline membranes for acid solution treatment by electrodialysis: Preparation, evaluation, and chemical calculation. J. Colloid Interface Sci. 2008, 320, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Farrokhzad, H.; Moghbeli, M.R.; Van Gerven, T.; Van der Bruggen, B. Surface modification of composite ion exchange membranes by polyaniline. React. Funct. Polym. 2015, 86, 161–167. [Google Scholar] [CrossRef]
- Reig, M.; Farrokhzad, H.; Van der Bruggen, B.; Gibert, O.; Cortina, J.L. Synthesis of a monovalent selective cation exchange membrane to concentrate reverse osmosis brines by electrodialysis. Desalination 2015, 375, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Berezina, N.P.; Kononenko, N.A.; Sytcheva, A.A.-R.; Loza, N.V.; Shkirskaya, S.A.; Hegman, N.; Pungor, A. Perfluorinated nanocomposite membranes modified by polyaniline: Electrotransport phenomena and morphology. Electrochim. Acta 2009, 54, 2342–2352. [Google Scholar] [CrossRef]
- Tan, S.; Bélanger, D. Characterization and transport properties of Nafion/Polyaniline composite membranes. J. Phys. Chem. B 2005, 109, 23480–23490. [Google Scholar] [CrossRef]
- Ben Jadi, S.; El Guerraf, A.; Kiss, A.; El Azrak, A.; Bazzaoui, E.A.; Wang, R.; Martins, J.I.; Bazzaoui, M. Analyses of scanning electrochemical microscopy and electrochemical impedance spectroscopy in direct methanol fuel cells: Permeability resistance and proton conductivity of polyaniline modified membrane. J. Solid State Electrochem. 2020, 24, 1551–1565. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Loza, N.V.; Shkirskaya, S.A.; Falina, I.V.; Khanukaeva, D.Y. Influence of conditions of polyaniline synthesis in perfluorinated membrane on electrotransport properties and surface morphology of composites. J. Solid State Electrochem. 2015, 19, 2623–2631. [Google Scholar] [CrossRef]
- Loza, N.V.; Dolgopolov, S.V.; Kononenko, N.A.; Andreeva, M.A.; Korshikova, Y.S. Effect of surface modification of perfluorinated membranes with polyaniline on their polarization behavior. Rus. J. Electrochem. 2015, 51, 538–545. [Google Scholar] [CrossRef]
- Andreeva, M.; Loza, N.; Kutenko, N.; Kononenko, N. Polymerization of aniline in perfluorinated membranes under conditions of electrodiffusion of monomer and oxidizer. J. Solid State Electrochem. 2020, 24, 101–110. [Google Scholar] [CrossRef]
- Loza, N.V.; Falina, I.V.; Kononenko, N.A.; Kudashova, D.S. Some aspects of polyaniline template synthesis within and on the surface of perfluorinated cation exchange membrane. Synth. Met. 2020, 261, 116292. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B. Perfluorinated ion-exchange membranes. Polym. Sci. A 2013, 55, 674–698. [Google Scholar] [CrossRef]
- Mahato, N.; Jang, H.; Dhyani, A.; Cho, S. Recent progress in conducting polymers for hydrogen storage and fuel cell applications. Polymers 2020, 12, 2480. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite polymers development and application for polymer electrolyte membrane technologies-a review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef] [Green Version]
- Parshina, A.; Kolganova, T.; Safronova, E.; Osipov, A.; Lapshina, E.; Yelnikova, A.; Bobreshova, O.; Yaroslavtsev, A. Perfluorosulfonic acid membranes thermally treated and modified by dopants with proton-acceptor properties for asparaginate and potassium ions determination in pharmaceuticals. Membranes 2019, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, S.V.; Zhelonkina, E.A.; Kononova, T.V. Effect of chromium compounds on the properties of ion-exchange membranes. Pet. Chem. 2013, 53, 494–499. [Google Scholar] [CrossRef]
- Shishkina, S.V.; Pechenkina, E.S.; Dyukov, A.V. Transport properties of anion-exchange membranes: Effect of the formation of complexes. Rus. J. Electrochem. 2006, 42, 1310–1318. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Pis’Menskii, V.F.; Demina, O.A.; Novak, L. Effect of concentration polarization on electrodialytic concentrating of dilute NaCl and NH4NO3 solutions. Rus. J. Electrochem. 2013, 49, 563–570. [Google Scholar] [CrossRef]
- Melnikov, S.; Sheldeshov, N.; Zabolotsky, V.; Loza, S.; Achoh, A. Pilot scale complex electrodialysis technology for processing a solution of lithium chloride containing organic solvents. Sep. Purif. Techol. 2017, 189, 74–81. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Fomenko, M.A.; Volfkovich, Y.M. Structure of perfluorinated membranes investigated by method of standard contact porosimetry. Adv. Colloid Interface Sci. 2015, 222, 425–435. [Google Scholar] [CrossRef]
- Berezina, N.P.; Timofeev, S.V.; Kononenko, N.A. Effect of conditioning techniques of perfluorinated sulphocationic membranes on their hydrophylic and electrotransport properties. J. Membr. Sci. 2002, 209, 509–518. [Google Scholar] [CrossRef]
- Loza, N.V.; Loza, S.A.; Kononenko, N.A. The Characteristics Changing Method of Electrodialyzer with Alternating Cation-Exchange and Anion-Exchange Membranes. RU Patent No. 2566415, 18 July 2014. [Google Scholar]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: Properties vs structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Zabolotsky, V.I.; Pismenskaya, N.D.; Lactionov, E.V.; Nikonenko, V.V. Prediction of the behavior of long electrodialysis desalination channels through testing short channels. Desalination 1996, 107, 245–250. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V. Effect of structural membrane inhomogeneity on transport properties. J. Membr. Sci. 1993, 79, 181–198. [Google Scholar] [CrossRef]
- Demina, O.A.; Kononenko, N.A.; Falina, I.V. New approach to the characterization of ion exchange membranes using a set of model parameters. Petrol. Chem. 2014, 54, 515–525. [Google Scholar] [CrossRef]
- Falina, I.V.; Demina, O.A.; Kononenko, N.A.; Annikova, L.A. Influence of inert components on the formation of conducting channels in ion-exchange membranes. J. Solid State Electrochem. 2017, 21, 767–775. [Google Scholar] [CrossRef]
- Ibanez, R.; Stamatialis, D.F.; Wessling, M. Role of membrane surface in concentration polarization at cation exchange membranes. J. Membr. Sci. 2004, 239, 119–128. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Dolgopolov, S.V.; Loza, N.V.; Shel’deshov, N.V. Effects of pH variation in solutions under the polarization conditions of the MF-4SK membrane with surface modified by polyaniline. Rus. J. Electrochem. 2015, 51, 19–24. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Loza, N.V.; Andreeva, M.A.; Shkirskaya, S.A.; Dammak, L. Influence of electric field during the chemical synthesis of polyaniline on the surface of heterogeneous sulfonated cation-exchange membranes on the their structure and properties. Membr. Membr. Technol. 2019, 1, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Zabolotsky, V.I.; Achoh, A.R.; Lebedev, K.A.; Melnikov, S.S. Permselectivity of bilayered ion-exchange membranes in ternary electrolyte. J. Membr. Sci. 2020, 608, 118152. [Google Scholar] [CrossRef]
- Robinson, R.A.; Stokes, R.H. Electrolyte Solutions, 2nd ed.; Dover Publications Inc.: New York, NY, USA, 2002; p. 571. ISBN 0-486-42225-9. [Google Scholar]
- Dong, T.; Yao, J.; Wang, Y.; Luo, T.; Han, L. On the permselectivity of di- and mono-valent cations: Influence of applied current density and ionic species concentration. Desalination 2020, 488, 114521. [Google Scholar] [CrossRef]
- Roghmans, F.; Evdochenko, E.; Martí-Calatayud, M.C.; Garthe, M.; Tiwari, R.; Walther, A.; Wessling, M. On the permselectivity of cation-exchange membranes bearing an ion selective coating. J. Membr. Sci. 2020, 600, 117854. [Google Scholar] [CrossRef]














| Membrane | IEC, mmol/gsw | Water Content, % | Density, gsw/cm3 | Specific Water Content, mol H2O/ mol SO3− |
|---|---|---|---|---|
| MK-40 | 1.54 ± 0.04 | 37 | 1.174 | 13 |
| MK-40/PANI | 1.55 ± 0.04 | 40 | 1.12 | 14 |
| MF-4SK | 0.68 ± 0.04 | 20 | 1.70 | 16 |
| MF-4SK/PANI | 0.70 ± 0.04 | 21 | 1.68 | 17 |
| Membrane | Electrolyte | f2 | α | a | b | c | d |
|---|---|---|---|---|---|---|---|
| MK-40 | HCl | 0.18 | 0.38 | 0.40 | 0.59 | 0.011 | 0.43 |
| MK-40/PANI | 0.22 | 0.59 | 0.27 | 0.65 | 0.078 | 0.54 | |
| MF-4SK | 0.08 | 0.31 | 0.24 | 0.76 | 2.9 × 10−4 | 0.34 | |
| MF-4SK/PANI | 0.12 | 0.46 | 0.24 | 0.75 | 0.0102 | 0.47 | |
| MK-40 | NaCl | 0.15 | 0.53 | 0.23 | 0.74 | 0.026 | 0.52 |
| MK-40/PANI | 0.21 | 0.51 | 0.32 | 0.64 | 0.045 | 0.50 | |
| MF-4SK | 0.05 | 0.40 | 0.12 | 0.88 | 6.0 × 10−4 | 0.42 | |
| MF-4SK/PANI | 0.12 | 0.51 | 0.20 | 0.79 | 0.014 | 0.51 | |
| MK-40 | CaCl2 | 0.19 | 0.41 | 0.38 | 0.61 | 0.016 | 0.45 |
| MK-40/PANI | 0.25 | 0.38 | 0.51 | 0.47 | 0.026 | 0.44 | |
| MF-4SK | 0.03 | 0.53 | 0.051 | 0.95 | 12 × 10−4 | 0.53 | |
| MF-4SK/PANI | 0.14 | 0.43 | 0.29 | 0.70 | 0.010 | 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falina, I.; Loza, N.; Loza, S.; Titskaya, E.; Romanyuk, N. Permselectivity of Cation Exchange Membranes Modified by Polyaniline. Membranes 2021, 11, 227. https://doi.org/10.3390/membranes11030227
Falina I, Loza N, Loza S, Titskaya E, Romanyuk N. Permselectivity of Cation Exchange Membranes Modified by Polyaniline. Membranes. 2021; 11(3):227. https://doi.org/10.3390/membranes11030227
Chicago/Turabian StyleFalina, Irina, Natalia Loza, Sergey Loza, Ekaterina Titskaya, and Nazar Romanyuk. 2021. "Permselectivity of Cation Exchange Membranes Modified by Polyaniline" Membranes 11, no. 3: 227. https://doi.org/10.3390/membranes11030227