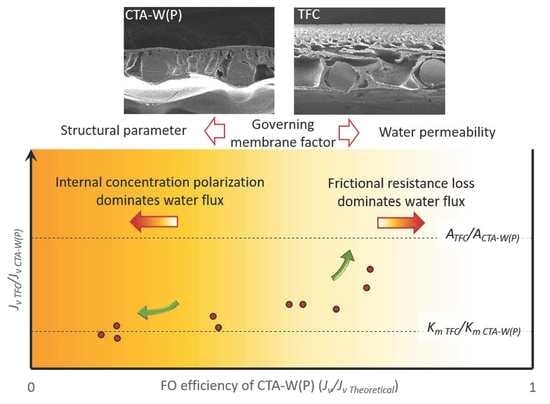
Insights into the Influence of Membrane Permeability and Structure on Osmotically-Driven Membrane Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membranes and Chemicals
2.2. Membrane Characterization
2.2.1. Characterization of Membrane Structure, Morphology and Chemical Property
2.2.2. Measurement of Membrane Permeability
2.2.3. Evaluation of OM Performance
3. Results and Discussions
3.1. Structure, Morphology, and Chemical Property of Membranes
3.2. Intrinsic Separation Property of Membranes
3.3. Membrane Performance in Osmotically-Driven Processes
3.3.1. FO Performance of Membranes
3.3.2. Anti-Scaling Performance of Membranes
3.3.3. PRO Performance of Membranes
4. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A. Structure of CTA-W and CTA-NW Membranes

Appendix B. Tensile Strength Tests

Appendix C. Property and Performance of Commercial FO Membranes
| Membrane ID | Manufacturer | Membrane Structure | Membrane Material | Membrane Property | FO Performance and Testing Conditions | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (L/(m2 h bar)) | BNaCl (L/(m2 h)) | BNaCl/A (bar) | S (mm) | Jv (L/(m2 h)) | Js (g/(m2 h)) | Js/Jv (g/L) | Membrane Orientation | Feed Solution | Draw Solution | ||||||
| 1 | TFC | HTI | TFC flat-sheet membrane with mesh | PA/PSf/PET | 2.56 ± 0.08 | 0.63 ± 0.21 | 0.25 ± 0.08 | 1.14 ± 0.12 | 15.82 ± 2.01 | 4.54 ± 2.44 | 0.28 ± 0.13 | AL-DS | 10 mM NaCl | 0.5 M NaCl | Current work |
| 8.51 ± 0.25 | 2.74 ± 0.99 | 0.32 ± 0.11 | AL-FS | 10 mM NaCl | 0.5 M NaCl | ||||||||||
| 8.07 ± 1.81 | N.A. | N.A. | AL-DS | 0.5 M NaCl | 2 M NaCl | ||||||||||
| 7.35 ± 0.83 | N.A. | N.A. | AL-FS | 0.5 M NaCl | 2 M NaCl | ||||||||||
| 2 | CTA-W(P) | HTI | Asymmetric flat-sheet membrane with mesh | CTA/PET | 1.06 ± 0.07 | 0.67 ± 0.24 | 0.65 ± 0.27 | 0.73 ± 0.31 | 14.85 ± 0.34 | 12.80 ± 1.88 | 0.86 ± 0.14 | AL-DS | 10 mM NaCl | 0.5 M NaCl | |
| 8.88 ± 0.41 | 7.05 ± 0.22 | 0.80 ± 0.06 | AL-FS | 10 mM NaCl | 0.5 M NaCl | ||||||||||
| 14.65 ± 0.64 | N.A. | N.A. | AL-DS | 0.5 M NaCl | 2 M NaCl | ||||||||||
| 11.90 ± 0.69 | N.A. | N.A. | AL-FS | 0.5 M NaCl | 2 M NaCl | ||||||||||
| 3 | CTA-W | HTI | Asymmetric flat-sheet membrane with mesh | CTA/PET | 0.41 ± 0.01 | 0.15 ± 0.01 | 0.38 ± 0.02 | 0.82 ± 0.39 | 7.07 ± 0.32 | 3.78 ± 0.62 | 0.54 ± 0.10 | AL-DS | 10 mM NaCl | 0.5 M NaCl | |
| 5.16 ± 0.17 | 1.94 ± 1.24 | 0.37 ± 0.24 | AL-FS | 10 mM NaCl | 0.5 M NaCl | ||||||||||
| 9.71 ± 0.85 | N.A. | N.A. | AL-DS | 0.5 M NaCl | 2 M NaCl | ||||||||||
| 8.32 ± 0.05 | N.A. | N.A. | AL-FS | 0.5 M NaCl | 2 M NaCl | ||||||||||
| 4 | CTA-NW | HTI | Asymmetric flat-sheet membrane with nonwoven fabric | CTA/PET | 0.53 ± 0.03 | 0.16 ± 0.04 | 0.31 ± 0.06 | 1.77 ± 0.20 | 7.03 ± 0.37 | 2.37 ± 1 | 0.35 ± 0.01 | AL-DS | 10 mM NaCl | 0.5 M NaCl | |
| 3.54 ± 0.21 | 1.03 ± 0.27 | 0.29 ± 0.09 | AL-FS | 10 mM NaCl | 0.5 M NaCl | ||||||||||
| 4.41 ± 0.48 | N.A. | N.A. | AL-DS | 0.5 M NaCl | 2 M NaCl | ||||||||||
| 4.14 ± 0.23 | N.A. | N.A. | AL-FS | 0.5 M NaCl | 2 M NaCl | ||||||||||
| 5 | Oasys | Oasys Water | TFC flat-sheet membrane with fabric | PA/PSf/PET | 4.25 ± 0.04 | 0.38 ± 0.11 | 0.089 a | 0.483 ± 0.079 | ~32 b | ~7 b | 0.22 a | AL-DS | DI water | ~0.5 M NaCl | [28] |
| ~15 b | ~2 b | 0.13 a | AL-FS | DI water | ~0.5 M NaCl | ||||||||||
| 6 | Porifera | Porifera | TFC flat-sheet membrane with mesh | PA-based | 2.2 | 0.50 | 0.23 a | 0.344 | 15.6 ± 0.5 | 10.30 a | 0.66 ± 0.02 | AL-FS | DI water | 0.5M NaCl | [31] |
| 7 | WJ-FO | Woongjin Chemical | TFC flat-sheet membrane | PA-based | 2.7 ± 1.12 | 0.83 ± 0.47 | 0.322 | 0.55 ± 0.21 | ~11 b | 16.5 a | 1.5 | AL-FS | DI water | 0.5 M NaCl | [30] |
| 8 | TCK-N | Toray Chemical Korea | TFC flat-sheet membrane with nonwoven fabric | PA/PSf/PET | 6.601 | 1.19 | 0.18 a | 0.461 | ~56 b | ~34 b | 0.61 a | AL-DS | DI water | 1 M NaCl | [29] |
| ~28 b | ~15 b | 0.54 a | AL-FS | DI water | 1 M NaCl | ||||||||||
| 9 | TCK-W | Toray Chemical Korea | TFC flat-sheet membrane with mesh | PA/PSf/PET | 5.362 | 0.95 | 0.18 a | 0.266 | ~64 b | ~25 b | 0.39 a | AL-DS | DI water | 1 M NaCl | |
| ~38 b | ~12 b | 0.32 a | AL-FS | DI water | 1 M NaCl | ||||||||||
| 10 | Aquaporin Inside | Aquaporin A/S, Denmark | TFC flat-sheet membrane | Aquaporin-PA-based | 0.52 ± 0.06 | 0.09 | 0.17 a | 0.825 | ~8 b | ~3 b | ~0.4 b | AL-DS | DI water | 0.5 M NaCl | [32] |
| ~5 b | ~1.5 b | ~0.32 b | AL-FS | DI water | 0.5 M NaCl | ||||||||||
| 11 | HF-A | Toyobo | Asymmetric hollow fiber membrane | CTA | 0.266 | 0.08 | 0.30 | 1.024 | ~6 b | N.A. | N.A. | AL-DS | DI water | 0.6 M NaCl | [25] |
| ~3 b | N.A. | N.A. | AL-FS | DI water | 0.6 M NaCl | ||||||||||
| 12 | HF-B | Toyobo | Asymmetric hollow fiber membrane | CTA | 0.291 | 0.02 | 0.069 | 0.724 | ~6 b | N.A. | N.A. | AL-DS | DI water | 0.6 M NaCl | |
| ~4 b | N.A. | N.A. | AL-FS | DI water | 0.6 M NaCl | ||||||||||
| 13 | HF-C | Toyobo | Asymmetric hollow fiber membrane | CTA | 0.545 | 0.04 | 0.073 | 0.639 | ~10 b | N.A. | N.A. | AL-DS | DI water | 0.6 M NaCl | |
| ~6 b | N.A. | N.A. | AL-FS | DI water | 0.6 M NaCl | ||||||||||
Appendix D. FO Performance of Membranes

References
- Xue, W.; Tobino, T.; Nakajima, F.; Yamamoto, K. Seawater-driven forward osmosis for enriching nitrogen and phosphorous in treated municipal wastewater: Effect of membrane properties and feed solution chemistry. Water Res. 2015, 69, 120–130. [Google Scholar] [CrossRef]
- Liyanaarachchi, S.; Jegatheesan, V.; Muthukumaran, S.; Gray, S.; Shu, L. Mass balance for a novel RO/FO hybrid system in seawater desalination. J. Membr. Sci. 2016, 501, 199–208. [Google Scholar] [CrossRef]
- Phuntsho, S.; Shon, H.K.; Hong, S.; Lee, S.; Vigneswaran, S. A novel low energy fertilizer driven forward osmosis desalination for direct fertigation: Evaluating the performance of fertilizer draw solutions. J. Membr. Sci. 2011, 375, 172–181. [Google Scholar] [CrossRef]
- Blandin, G.; Ferrari, F.; Lesage, G.; Le-Clech, P.; Héran, M.; Martinez-Lladó, X. Forward Osmosis as Concentration Process: Review of Opportunities and Challenges. Membranes 2020, 10, 284. [Google Scholar] [CrossRef]
- Coday, B.D.; Xu, P.; Beaudry, E.G.; Herron, J.; Lampi, K.; Hancock, N.T.; Cath, T.Y. The sweet spot of forward osmosis: Treatment of produced water, drilling wastewater, and other complex and difficult liquid streams. Desalination 2014, 333, 23–35. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Li, Z.; Sarp, S.; Bucs, S.; Amy, G.; Vrouwenvelder, J.S. Forward osmosis niches in seawater desalination and wastewater reuse. Water Res. 2014, 66, 122–139. [Google Scholar] [CrossRef]
- Ferrari, F.; Pijuan, M.; Rodriguez-Roda, I.; Blandin, G. Exploring Submerged Forward Osmosis for Water Recovery and Pre-Concentration of Wastewater before Anaerobic Digestion: A Pilot Scale Study. Membranes 2019, 9, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phuntsho, S.; Hong, S.; Elimelech, M.; Shon, H.K. Forward osmosis desalination of brackish groundwater: Meeting water quality requirements for fertigation by integrating nanofiltration. J. Membr. Sci. 2013, 436, 1–15. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.R.D.; Tang, C.Y.; Le-Clech, P. Opportunities to reach economic sustainability in forward osmosis–reverse osmosis hybrids for seawater desalination. Desalination 2015, 363, 26–36. [Google Scholar] [CrossRef]
- Altaee, A.; Braytee, A.; Millar, G.J.; Naji, O. Energy efficiency of hollow fibre membrane module in the forward osmosis seawater desalination process. J. Membr. Sci. 2019, 587, 117165. [Google Scholar] [CrossRef]
- Choi, B.G.; Zhan, M.; Shin, K.; Lee, S.; Hong, S. Pilot-scale evaluation of FO-RO osmotic dilution process for treating wastewater from coal-fired power plant integrated with seawater desalination. J. Membr. Sci. 2017, 540, 78–87. [Google Scholar] [CrossRef]
- Wang, X.; Chang, V.W.C.; Tang, C.Y. Osmotic membrane bioreactor (OMBR) technology for wastewater treatment and reclamation: Advances, challenges, and prospects for the future. J. Membr. Sci. 2016, 504, 113–132. [Google Scholar] [CrossRef]
- Werner, C.M.; Logan, B.E.; Saikaly, P.E.; Amy, G.L. Wastewater treatment, energy recovery and desalination using a forward osmosis membrane in an air-cathode microbial osmotic fuel cell. J. Membr. Sci. 2013, 428, 116–122. [Google Scholar] [CrossRef]
- Helfer, F.; Lemckert, C.; Anissimov, Y.G. Osmotic power with Pressure Retarded Osmosis: Theory, performance and trends—A review. J. Membr. Sci. 2014, 453, 337–358. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Jia, J.; Miao, H.; Ruan, W.; Wang, X. Performance Improvement and Biofouling Mitigation in Osmotic Microbial Fuel Cells via In Situ Formation of Silver Nanoparticles on Forward Osmosis Membrane. Membranes 2020, 10, 122. [Google Scholar] [CrossRef]
- Rastogi, N.K. Opportunities and Challenges in Application of Forward Osmosis in Food Processing. Crit. Rev. Food Sci. 2016, 56, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, V.; Marczak, L.D.F.; Tessaro, I.C. Membrane concentration of liquid foods by forward osmosis: Process and quality view. J. Food Eng. 2012, 111, 483–489. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Tang, C.Y.; She, Q.; Lay, W.C.L.; Wang, R.; Fane, A.G. Coupled effects of internal concentration polarization and fouling on flux behavior of forward osmosis membranes during humic acid filtration. J. Membr. Sci. 2010, 354, 123–133. [Google Scholar] [CrossRef]
- Tang, C.Y.; She, Q.; Lay, W.C.L.; Wang, R.; Field, R.; Fane, A.G. Modeling double-skinned FO membranes. Desalination 2011, 283, 178–186. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.; Wang, Y.N.; Wang, R.; Tang, C.Y. Comparison of NF-like and RO-like thin film composite osmotically-driven membranes-Implications for membrane selection and process optimization. J. Membr. Sci. 2013, 427, 460–471. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.; Tang, C.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Shibuya, M.; Yasukawa, M.; Takahashi, T.; Miyoshi, T.; Higa, M.; Matsuyama, H. Effect of operating conditions on osmotic-driven membrane performances of cellulose triacetate forward osmosis hollow fiber membrane. Desalination 2015, 362, 34–42. [Google Scholar] [CrossRef]
- Kumano, A.; Marui, K.; Terashima, Y. Hollow fiber type PRO module and its characteristics. Desalination 2016, 389, 149–154. [Google Scholar] [CrossRef]
- Ren, J.; McCutcheon, J.R. A new commercial thin film composite membrane for forward osmosis. Desalination 2014, 343, 187–193. [Google Scholar] [CrossRef]
- Arena, J.T.; Manickam, S.S.; Reimund, K.K.; Brodskiy, P.; McCutcheon, J.R. Characterization and Performance Relationships for a Commercial Thin Film Composite Membrane in Forward Osmosis Desalination and Pressure Retarded Osmosis. Ind. Eng. Chem. Res. 2015, 54, 11393–11403. [Google Scholar] [CrossRef]
- Nguyen, T.P.N.; Jun, B.M.; Lee, J.H.; Kwon, Y.N. Comparison of integrally asymmetric and thin film composite structures for a desirable fashion of forward osmosis membranes. J. Membr. Sci. 2015, 495, 457–470. [Google Scholar] [CrossRef]
- Sahebi, S.; Phuntsho, S.; Tijing, L.; Han, G.; Han, D.S.; Abdel-Wahab, A.; Shon, H.K. Thin-film composite membrane on a compacted woven backing fabric for pressure assisted osmosis. Desalination 2017, 406, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Blandin, G.; Vervoort, H.; D’Haese, A.; Schoutteten, K.; Bussche, J.V.; Vanhaecke, L.; Myat, D.T.; Le-Clech, P.; Verliefde, A.R.D. Impact of hydraulic pressure on membrane deformation and trace organic contaminants rejection in pressure assisted osmosis (PAO). Process Saf. Environ. 2016, 102, 316–327. [Google Scholar] [CrossRef]
- Xia, L.L.; Andersen, M.F.; Helix-Nielsen, C.; McCutcheon, J.R. Novel Commercial Aquaporin Flat-Sheet Membrane for Forward Osmosis. Ind. Eng. Chem. Res. 2017, 56, 11919–11925. [Google Scholar] [CrossRef]
- Ye, W.; Lin, J.; Tækker Madsen, H.; Gydesen Søgaard, E.; Hélix-Nielsen, C.; Luis, P.; Van der Bruggen, B. Enhanced performance of a biomimetic membrane for Na2CO3 crystallization in the scenario of CO2 capture. J. Membr. Sci. 2016, 498, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; Alanezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent developments in forward osmosis membranes using carbon-based nanomaterials. Desalination 2020, 482, 114375. [Google Scholar] [CrossRef]
- Wu, W.; Shi, Y.; Liu, G.; Fan, X.; Yu, Y. Recent development of graphene oxide based forward osmosis membrane for water treatment: A critical review. Desalination 2020, 491, 114452. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Q.; Ge, Q. Recent advances in forward osmosis (FO) membrane: Chemical modifications on membranes for FO processes. Desalination 2017, 419, 101–116. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, K.Y.; Chung, T.S.; Jean, Y.C.; Chen, H. Molecular design of the cellulose ester-based forward osmosis membranes for desalination. Chem. Eng. J. 2011, 66, 2008–2018. [Google Scholar] [CrossRef]
- Su, J.; Yang, Q.; Teo, J.F.; Chung, T.S. Cellulose acetate nanofiltration hollow fiber membranes for forward osmosis processes. J. Membr. Sci. 2010, 355, 36–44. [Google Scholar] [CrossRef]
- Wang, R.; Shi, L.; Tang, C.Y.; Chou, S.; Qiu, C.; Fane, A.G. Characterization of novel forward osmosis hollow fiber membranes. J. Membr. Sci. 2010, 355, 158–167. [Google Scholar] [CrossRef]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. High performance thin-film composite forward osmosis membrane. Environ. Sci. Technol. 2010, 44, 3812–3818. [Google Scholar] [CrossRef] [PubMed]
- Bui, N.N.; McCutcheon, J.R. Hydrophilic nanofibers as new supports for thin film composite membranes for engineered osmosis. Environ. Sci. Technol. 2013, 47, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.X.; Liu, C.; Shi, L.; Wang, R. Composite forward osmosis hollow fiber membranes: Integration of RO- and NF-like selective layers for enhanced organic fouling resistance. J. Membr. Sci. 2015, 492, 147–155. [Google Scholar] [CrossRef]
- Setiawan, L.; Wang, R.; Shi, L.; Li, K.; Fane, A.G. Novel dual-layer hollow fiber membranes applied for forward osmosis process. J. Membr. Sci. 2012, 421, 238–246. [Google Scholar] [CrossRef]
- Saren, Q.; Qiu, C.Q.; Tang, C.Y. Synthesis and characterization of novel forward osmosis membranes based on layer-by-layer assembly. Environ. Sci. Technol. 2011, 45, 5201–5208. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chung, T.-S.; Qin, J.-J. Polybenzimidazole (PBI) nanofiltration hollow fiber membranes applied in forward osmosis process. J. Membr. Sci. 2007, 300, 6–12. [Google Scholar] [CrossRef]
- Qi, S.R.; Li, Y.; Zhao, Y.; Li, W.Y.; Tang, C.Y.Y. Highly Efficient Forward Osmosis Based on Porous Membranes-Applications and Implications. Environ. Sci. Technol. 2015, 49, 4690–4695. [Google Scholar] [CrossRef] [PubMed]
- Akther, N.; Phuntsho, S.; Chen, Y.; Ghaffour, N.; Shon, H.K. Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes. J. Membr. Sci. 2019, 584, 20–45. [Google Scholar] [CrossRef]
- She, Q.; Zhang, L.; Wang, R.; Krantz, W.B.; Fane, A.G. Pressure-retarded osmosis with wastewater concentrate feed: Fouling process considerations. J. Membr. Sci. 2017, 542, 233–244. [Google Scholar] [CrossRef]
- Loeb, S.; Titelman, L.; Korngold, E.; Freiman, J. Effect of porous support fabric on osmosis through a Loeb-Sourirajan type asymmetric membrane. J. Membr. Sci. 1997, 129, 243–249. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, J.; Tang, C.Y. Gypsum scaling during forward osmosis process—A direct microscopic observation study. Desalination Water Treat. 2016, 57, 3317–3327. [Google Scholar] [CrossRef]
- Wang, Y.N.; Järvelä, E.; Wei, J.; Zhang, M.; Kyllönen, H.; Wang, R.; Tang, C.Y. Gypsum scaling and membrane integrity of osmotically driven membranes: The effect of membrane materials and operating conditions. Desalination 2016, 377, 1–10. [Google Scholar] [CrossRef]
- She, Q.; Jin, X.; Tang, C.Y. Osmotic power production from salinity gradient resource by pressure retarded osmosis: Effects of operating conditions and reverse solute diffusion. J. Membr. Sci. 2012, 401, 262–273. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes: I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Giagnorio, M.; Ricceri, F.; Tagliabue, M.; Zaninetta, L.; Tiraferri, A. Hybrid Forward Osmosis–Nanofiltration for Wastewater Reuse: System Design. Membranes 2019, 9, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Gray, S.R. Gypsum scaling in forward osmosis: Role of membrane surface chemistry. J. Membr. Sci. 2016, 513, 250–259. [Google Scholar] [CrossRef]
- She, Q.; Hou, D.; Liu, J.; Tan, K.H.; Tang, C.Y. Effect of feed spacer induced membrane deformation on the performance of pressure retarded osmosis (PRO): Implications for PRO process operation. J. Membr. Sci. 2013, 445, 170–182. [Google Scholar] [CrossRef]










| Property | TFC | CTA-W(P) | CTA-W | CTA-NW | |
|---|---|---|---|---|---|
| Membrane material | PA/PSf/PET | CTA/PET | CTA/PET | CTA/PET | |
| Structural property | Thickness (µm) | 105 ± 4 | 71 ± 7 a | 61 ± 1 a (44.7 ± 14.1) b | 129 ± 6 (144 ± 24) b |
| Porosity (%) | 66 ± 1 | 62 ± 1 | 50 ± 1 (46 ± 1) b | 55 ± 1 (50 ± 2) b | |
| Structural parameter, S (mm) | 1.14 ± 0.12 | 0.73 ± 0.31 | 0.82 ± 0.39 (1.00 ± 0.54) b | 1.77 ± 0.20 (1.38 ± 0.26) b | |
| Surface property | Roughness, Rq (nm) | 69 ± 15 | 5 ± 1 | 5 ± 1 | 33 ± 18 |
| Contact angle (°) | 48 ± 3 | 63 ± 1 | 67 ± 2 (73 ± 2) b | 63 ± 4 (64 ± 2) b | |
| Mechanical property | Young’s modulus, E (MPa) | ||||
| With tension in axial direction | 158 ± 31 | 427 ± 34 | 384 ± 33 | 261 ± 70 (Isotropic) | |
| With tension in diagonal direction | 38 ± 3 | 152 ± 13 | 264 ± 14 | ||
| Tensile strain at break (%) | |||||
| With tension in axial direction | 67 ± 2 | 55 ± 4 | 65 ± 7 | 77 ± 11 (Isotropic) | |
| With tension in diagonal direction | 151 ± 14 | 175 ± 16 | 150 ± 12 | ||
| Tensile stress at break (MPa) | |||||
| With tension in axial direction | 54 ± 3 | 76 ± 9 | 92 ± 8 | 26 ± 5 (Isotropic) | |
| With tension in diagonal direction | 32 ± 1 | 76 ± 2 | 95 ± 4 | ||
| Intrinsic separation property | Water permeability, A (L/(m2 h bar)) | 2.56 ± 0.08 | 1.06 ± 0.07 | 0.41 ± 0.01 (0.33 ± 0.04) b | 0.53 ± 0.03 (0.46 ± 0.07) b |
| NaCl rejection (%) | 96.5 ± 0.9 | 88.1 ± 4.3 | 92.5 ± 0.3 (81.9) b,c | 93.9 ± 1.2 (92.4) b,c | |
| NaCl permeability, BNaCl (L/(m2 h)) | 0.63 ± 0.21 | 0.67 ± 0.24 | 0.15 ± 0.01 (0.14 ± 0.03) b | 0.16 ± 0.04 (0.10 ± 0.01) b | |
| BNaCl/A (bar) | 0.25 ± 0.08 | 0.65 ± 0.27 | 0.38 ± 0.02 (0.47 ± 0.12) b | 0.31 ± 0.06 (0.22 ± 0.03) b | |
| Na2SO4 rejection (%) | 98.9 ± 0.2 | 96.4 ± 1.8 | 99.0 ± 0.04 | 98.8 ± 0.01 | |
| Na2SO4 permeability, BNa2SO4 (L/(m2 h)) | 0.13 ± 0.02 | 0.17 ± 0.08 | 0.019 ± 0.0002 | 0.029 ± 0.001 | |
| BNa2SO4/A (bar) | 0.053 ± 0.008 | 0.17 ± 0.09 | 0.047 ± 0.001 | 0.055 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; She, Q.; Liu, X. Insights into the Influence of Membrane Permeability and Structure on Osmotically-Driven Membrane Processes. Membranes 2021, 11, 153. https://doi.org/10.3390/membranes11020153
Wei J, She Q, Liu X. Insights into the Influence of Membrane Permeability and Structure on Osmotically-Driven Membrane Processes. Membranes. 2021; 11(2):153. https://doi.org/10.3390/membranes11020153
Chicago/Turabian StyleWei, Jing, Qianhong She, and Xin Liu. 2021. "Insights into the Influence of Membrane Permeability and Structure on Osmotically-Driven Membrane Processes" Membranes 11, no. 2: 153. https://doi.org/10.3390/membranes11020153