A Review on Polymer Nanocomposites and Their Effective Applications in Membranes and Adsorbents for Water Treatment and Gas Separation
Abstract
:1. Polymer Nanocomposites for Environmental Applications
2. Polymer Nanocomposites Membranes
2.1. Polymer Nanocomposites Membranes for the Treatment of Wastewater
Mechanisms of Polymer Nanocomposites Membranes for Water Treatment
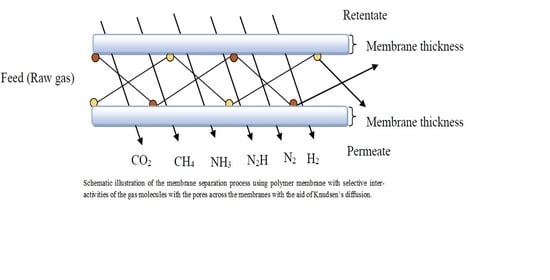
2.2. Polymer Nanocomposites Membranes for Gas Separation
Mechanisms of Gas Separation in Nanocomposite Membranes
- (1)
- Diverse components sorption from a feed in accordance with their partition coefficient amid the gas and polymer phase.
- (2)
- The separate components diffusion contained in the membrane phase in accordance with their activity gradients.
- (3)
- Components desorption from the membrane in the permeate gas phase.
2.3. Working Principles of Membranes
3. Polymer Nanocomposites as Adsorbent
3.1. Adsorbent from Nanocomposites Polymer for the Elimination of Metals Ions
3.2. Polymer Nanocomposites as Adsorbent for Dye Removal
3.2.1. Polymer Nanoparticle as Adsorbent for Dye Removal
3.2.2. Adsorbent Made from Polymer Carbon Nanotube for Dye Removal
3.3. Polymer Nanocomposites as Adsorbent for Separation of Gases
3.4. Mechanism of Adsorption
4. Features of the Transport and Dissolution of Substances in Polymer Nanocomposites
5. Patents in the Utilization of Polymer Nanocomposites Innovations for Environmental Applications and Future Work
5.1. Patents on Polymer Nanocomposites Membranes for Environmental Applications
5.2. Patents on Polymer Nanocomposites as Adsorbent for Environmental Applications
5.3. Future Work on Polymer Nanocomposites for Environmental Applications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, D.R.; Engelhard, M.H.; Johnson, G.E.; Laskin, J.; Lai, J.; Mueller, K.; Munusamy, P.; Thevuthasan, S.; Wang, H.; Washton, N.; et al. Surface characterization of nanomaterials and nanoparticles: Important needs and challenging opportunities. J. Vac. Sci. Technol. A 2013, 31, 1–34. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon nanotubes: A review on structure and their interaction with proteins. J. Chem. 2013, 676815, 1–18. [Google Scholar] [CrossRef]
- Jian, S.; Zhu, J.; Jiang, S.; Chen, S.; Fang, H.; Song, Y.; Duan, G.; Zhang, Y.; Hou, H. Nanofibers with diameter below one nanometer from electrospinning. RSC Adv. 2018, 8, 4794–4802. [Google Scholar] [CrossRef]
- Wang, X.; Pakdel, A.; Zhang, J.; Weng, Q.; Zhai, T.; Zhi, C.; Golberg, D.; Bando, Y. Large-surface-area BN nanosheets and their utilization in polymeric composites with improved thermal and dielectric properties. Nanoscale Res. Lett. 2012, 7, 622. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, W.; Jiang, D.; Zheng, L.; Li, X.; Wang, Z. Ultrathin mesoporous Co3O4 nanosheets with excellent photo-/thermo-catalytic activity. Mater. Chem. A 2016, 4, 105–112. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Inamuddin. Carbon nanotube‑based adsorbents for the removal of dyes from waters: A review. Environ. Chem. Lett. 2020, 18, 605–629. [Google Scholar] [CrossRef]
- Masciangioli, T.; Zhang, W.X. Environmental technologies at the nanoscale. Environ. Sci. Technol. 2003, 37, 102A–108A. [Google Scholar] [CrossRef] [Green Version]
- Thondavada, N.; Chokkareddy, R.; Naidu, N.V.; Redhi, G.G. Environmental science and engineering applications of polymer and nanocellulose-based nanocomposites. In Composites for Environmental Engineering; Scrivener Publishing LLC: Beverly, MA, USA, 2019; pp. 135–178. [Google Scholar]
- Wang, Z.; Mi, B. Environmental applications of 2D molybdenum disulfide (Mo) nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef]
- Yoon, M.; Hsiao, B.S.; Chu, B. Functional nanofibers for environmental applications. J. Mater. Chem. 2018, 18, 5326–5334. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Christian, P.; Von der Kammer, F.; Baalousha, M.; Hofmann, T. Nanoparticles: Structure, properties, preparation and behaviour in environmental media. Ecotoxicology 2008, 17, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Barrak, H.; Saied, T.; Chevallier, P.; Laroche, G.; M’nif, A.; Hamzaoui, A.H. Synthesis, characterization, and functionalization of ZnO nanoparticles by N- (trimethoxysilylpropyl) ethylenediamine triacetic acid (TMSEDTA): Investigation of the interactions between phloroglucinol and ZnO@TMSEDTA. Arab. J. Chem. 2016, 8, 4340–4347. [Google Scholar] [CrossRef] [Green Version]
- Vykhodets, V.B.; Kurennykh, T.E. Characterization of the defect structure of oxide nanoparticles with the use of deuterium probes. RSC Adv. 2020, 10, 3837–3843. [Google Scholar] [CrossRef]
- Qui, H.; Yang, J. Structure and properties of carbon nanotubes. In Industrial Application of Carbon Nanotube; Elsevier: Amsterdam, The Netherlands, 2017; pp. 47–69. [Google Scholar]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R Report 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Ebbesen, T.W. Nanometre-size tubes of carbon. Rep. Prog. Phys. 1997, 60, 1025–1062. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, C.; Leung, C.K.Y.; Li, Z. Graphene oxide modified strain hardening cementitious composites with enhanced mechanical and thermal properties by incorporating ultra-fine phase change materials. Cem. Concr. Compos. 2019, 98, 83–94. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Ouyang, D. Effect of graphene oxide on mechanical properties of cement mortar and its strengthening mechanism. Materials 2019, 12, 3753. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Deng, C.; Lu, S.; Yang, Y.; Guan, G.; Liu, Z.; Yu, Q. Enhanced mechanical property, thermal and electrical conductivity of natural rubber/graphene nanosheets nanocomposites. Polym. Nanocomp. 2020, 41, 1299–1309. [Google Scholar] [CrossRef]
- Dastidar, D.G.; Chakrabarti, G. Thermoresponsive drug delivery systems, characterizations and applications. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Chen, H.; Di, J.; Wang, N.; Dong, H.; Wu, J.; Zhao, Y.; Yu, J.; Jiang, L. Fabrication of hierarchically porous inorganic nanofibers by a general microemulsion electrospinning approach. Small 2011, 7, 1779–1783. [Google Scholar] [CrossRef]
- Ali, N.S.; Gharehaghaji, A.A. How porous nanofibers have enhanced the engineering of advanced materials: A review. J. Text. Polym. 2017, 5, 57–72. [Google Scholar]
- Agboola, O.; Popoola, P.; Sadiku, R.; Sanni, S.E.; Fayomi, S.O.; Fatoba, O.S. Nanotechnology in wastewater and the capacity of nanotechnology for sustainability. In Environmental Chemistry for a Sustainable World; Springer Nature Switzerland: Cham, Switzerland, 2020; Volume 27, pp. 1–34. [Google Scholar]
- Tong, T.; Elimelech, M. The global rise of zero liquid discharge for wastewater management: Drivers, technologies, and future directions. Critical review. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Elbahri, M. Nanocomposite electrospun nanofiber membranes for environmental remediation: Review. Materials 2014, 7, 1017–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Lv, L.; Pan, B.; Zhang, W.; Zhang, S.; Zhang, Q. Polymer-supported nanocomposite for environmental application: A review. Chem. Eng. J. 2011, 170, 381–394. [Google Scholar] [CrossRef]
- Sinsawat, A.; Anderson, K.L.; Vaia, R.A.; Farmer, B.L. Influence of polymer matrix composition and architecture on polymer nanocomposite formation: Coarse grained molecular dynamics simulation. J. Polym. Sci. B Polym. Phys. 2003, 41, 3272–3284. [Google Scholar] [CrossRef]
- Sahoo, T.R. Polymer nanocomposites for environmental applications. In Properties and Applications of Polymer Nanocomposites—Clay and Carbon Based Polymer Nanocomposites; Springer Verlag GmbH: Heidelberg, Germany, 2017; p. 77. [Google Scholar]
- El-Kafrawy, A.F.; El-Saeed, S.M.; Farag, R.K.; El-Saied, A.L.; Abdel-Raouf, M.E. Adsorbents based on natural polymers for removal of some heavy metals from aqueous solution. Egyptian J. Pet. 2017, 26, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar] [CrossRef]
- Vatanpour, V.; Safarpour, M. Carbon-Based polymer nanocomposite membranes for desalination. In Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 234–281. [Google Scholar]
- Ying, Y.; Ying, W.; Li, Q.; Meng, D.; Ren, G.; Yan, R.; Peng, X. Recent advances of nanomaterial- based membrane for water purification. Appl. Mater. Today 2017, 7, 144–158. [Google Scholar] [CrossRef]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of nanocomposite membranes for water treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Aani, S.; Wright, C.J.; Atieh, M.A.; Hilal, N. Engineering nanocomposite membranes: Addressing current challenges and future opportunities. Desalination 2017, 401, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Hilal, N.; Ismail, A.F.; Wright, C. Membrane Fabrication; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Wang, P.; Ma, J.; Shi, F.; Ma, Y.; Wang, Z.; Zhao, X. Behaviors and effects of differing dimensional nanomaterials in water filtration membranes through the classical phase inversion process: A review. Ind. Eng. Chem. Res. 2013, 52, 10355–10363. [Google Scholar] [CrossRef]
- Kim, E.-S.; Hwang, G.; Gamal El-Din, M.; Liu, Y. Development of nanosilver and multiwalled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J. Membr. Sci. 2012, 394, 37–48. [Google Scholar] [CrossRef]
- Sathish, K.R.; Arthanareeswaran, G.; Paul, D.; Kweon, J.H. Modification methods of polyethersulfone membranes for minimizing fouling-Review. Membr. Water Treat. 2015, 6, 323–337. [Google Scholar]
- Wu, H.; Tang, B.; Wu, P. MWNTs/polyester thin film nanocomposite membrane: An approach to overcome the trade-off effect between permeability and selectivity. J. Phys. Chem. C 2010, 114, 16395–16400. [Google Scholar] [CrossRef]
- Brunet, L.; Lyon, D.Y.; Zodrow, K.; Rouch, J.C.; Caussat, B.; Serp, P.; Remigy, J.C.; Wiesner, M.R.; Alvarez, P.J.J. Properties of membranes containing semi-dispersed carbon nanotubes. Environ. Eng. Sci. 2008, 25, 565–576. [Google Scholar] [CrossRef]
- Kim, S.; Jinschek, J.R.; Chen, H.; Sholl, D.S.; Marand, E. Scalable fabrication of carbon nanotube/polymer nanocomposite membranes for high flux gas transport. Nano Lett. 2007, 7, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- Dumée, L.; Sears, K.; Schü, J.; Finn, N.; Duke, M.; Gray, S. Carbon nanotube based composite membranes for water desalination by membrane distillation. Desalin. Water Treat. 2010, 17, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Maphutha, S.; Moothi, K.; Meyyappan, M.; Iyuke, S.E. A carbon nanotube-infused polysulfone membrane with polyvinyl alcohol layer for treating oil-containing wastewater. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-F.; Chen, H.-Y.; Surapathi, A.; Taylor, M.G.; Shao, X.; Marand, E.; Johnson, J.K. Zwitterion functionalized carbon nanotube/polyamide nanocomposite membranes for water desalination. ACS Nano 2013, 7, 5308–5319. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, L.; Jia, N.; Wang, R.; Liu, L.; Gao, C. Polyphenol-metal manipulated nanohybridization of CNT membranes with FeOOH nanorods for high-flux, antifouling and self-cleaning oil/water separation. J. Membr. Sci. 2020, 600, 117857. [Google Scholar] [CrossRef]
- Akhavan, O.; Abdolahad, M.; Abdi, Y.; Mohajerzadeh, S. Silver nanoparticles within vertically aligned multi-wall carbon nanotubes with open tips for antibacterial purposes. J. Mater. Chem. 2011, 21, 387–393. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Bai, R.; Wee, K.-H.; Liu, C.; Tang, S.-L. Membrane surfaces immobilized with ionic or reduced silver and their anti-biofouling performances. J. Membr. Sci. 2010, 363, 278–286. [Google Scholar] [CrossRef]
- Sile-Yuksel, M.; Tas, B.; Koseoglu-Imer, D.Y.; Koyuncu, I. Effect of silver nanoparticle (AgNP) location in nanocomposite membrane matrix fabricated with different polymer type on antibacterial mechanism. Desalination 2014, 347, 120–130. [Google Scholar] [CrossRef]
- Haider, M.S.; Shao, G.N.; Imran, S.M.; Park, S.S.; Abbas, N.; Tahir, M.S.; Hussain, M.; Bae, W.; Kim, H.T. Aminated polyethersulfone-silver nanoparticles (AgNPs-APES) composite membranes with controlled silver ion release for antibacterial and water treatment applications. Mater. Sci. Eng. C 2016, 62, 732–745. [Google Scholar] [CrossRef]
- Biswas, P.; Bandyopadhyaya, R. Biofouling prevention using silver nanoparticle impregnated polyethersulfone (PES) membrane: E. coli cell-killing in a continuous cross-flow membrane module. J. Colloid Interface Sci. 2017, 491, 13–26. [Google Scholar] [CrossRef]
- Marino, A.F.T.; Boerrigter, M.; Faccini, M.; Chaumette, C.; Arockiasamy, L.; Bundschuh, J. Photocatalytic activity and synthesis procedures of TiO2 nanoparticles for potential applications in membranes. In Application of Nanotechnology in Membranes for Water Treatment; Figoli, J.B.A., Hoinkis, J., Altinkaya, S.A., Eds.; CRC Press, Taylor & Francis Group: Abingdon, UK, 2017. [Google Scholar]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Ebert, K.; Fritsch, D.; Koll, J.; Tjahjawiguna, C. Influence of inorganic fillers on the compaction behavior of porous polymer based membranes. J. Membr. Sci. 2004, 233, 71–78. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zinadini, S.; Vatanpour, V. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J. Membr. Sci. 2011, 380, 155–162. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, S.Y.; Sohn, B.H.; Park, T.H. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composition (TFC) membrane as an approach to solve bio-fouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Madaeni, S.S. Nanofiltration membranes. In Encyclopedia of Membranes; Droli, E., Giorno, L., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Indust. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Berber, M.R. Current advances of polymer composites for water treatment and desalination. J. Chem. 2020, 2020, 7608423. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.Z.; Engtrakul, C.; Bischoff, B.L.; Lu, M.; Alemseghed, M. Surface-Engineered inorganic nanoporous membranes for vapor and pervaporative separations of water–ethanol mixtures. Membranes 2018, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Salleh, W.N.W.; Ismail, A.F. Carbon membranes for gas separation processes: Recent progress and future perspective. J. Membr. Sci. Res. 2015, 1, 2–15. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: Preparation and experimental results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Matsuura, T.; Montazer-Rahmati, M.M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Moore, T.T.; Mahajan, R.; Vu, D.Q.; Koros, W.J. Hybrid membrane materials comprising organic polymers with rigid dispersed phases. AIChE J. 2004, 50, 311–321. [Google Scholar] [CrossRef]
- Noble, R.D.; Agrawal, R. Separations research needs for the 21st century. Ind. Eng. Chem. Res. 2005, 44, 2887–2892. [Google Scholar] [CrossRef]
- Cong, H.; Radosz, M.; Towler, B.F.; Shen, Y. Polymer-inorganic nanocomposite membranes for gas separation. Sep. Purif. Technol. 2007, 55, 281–291. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Kiadehi, D.A.; Jahanshahi, M.; Rahimpour, A.; Ghoreyshi, A.A. Fabrication and evaluation of functionalized nano-titanium dioxide (F-NanoTiO2)/polysulfone (PSf) nanocomposite membranes for gas separation. Iranian. J. Chem. Eng. 2014, 11, 40–49. [Google Scholar]
- Safaei, P.; Marjani, A.; Salimi, M. Mixed matrix membranes prepared from high impact polystyrene with dispersed TiO2 nanoparticles for gas separation. J. Nanostruct. 2016, 6, 74–79. [Google Scholar]
- Li, L.; Song, C.; Jang, D.; Wang, T. Preparation and enhanced gas separation performance of Carbon/Carbon nanotubes (C/CNTs) hybrid membranes. Sep. Purif. Technol. 2017, 188, 73–80. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Li, X.; Deng, M. Gas separation properties of poly(amide-6- b-ethylene oxide)/amino modified multi-walled carbon nanotubes mixed matrix membranes. J. Membr. Sci. 2014, 467, 41–47. [Google Scholar] [CrossRef]
- Rao, P.S.; Wey, M.; Tseng, H.; Kumar, I.A.; Weng, T. A comparison of carbon/nanotube molecular sieve membranes with polymer blend carbon molecular sieve membranes for the gas permeation application. Micropor. Mesopor. Mat. 2008, 113, 499–510. [Google Scholar] [CrossRef]
- Tseng, H.; Kumar, I.A.; Weng, T.; Lu, C.; Wey, M. Preparation and characterization of carbon molecular sieve membranes for gas separation-the effect of incorporated multi-wall carbon nanotubes. Desalination 2009, 240, 40–45. [Google Scholar] [CrossRef]
- Sanip, S.M.; Ismail, A.F.; Goh, P.S.; Soga, T.; Tanemura, M.; Yasuhiko, H. Gas separation properties of functionalized carbon nanotubes mixed matrix membranes. Sep. Purif. Technol. 2011, 78, 208–213. [Google Scholar] [CrossRef]
- Sun, H.; Wang, T.; Xu, Y.; Gao, W.; Li, P.; Niu, Q.J. Fabrication of polyimide and functionalized multi-walled carbon nanotubes mixed matrix membranes by in-situ polymerization for CO2 separation. Sep. Purif. Technol. 2017, 177, 327–336. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Fu, Y.-J.; Hu, C.-C.; Lin, D.-W.; Tsai, H.-A.; Huang, S.-H.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. Adjustable microstructure carbon molecular sieve membranes derived from thermally stable polyetherimide/polyimide blends for gas separation. Carbon 2017, 113, 10–17. [Google Scholar] [CrossRef]
- Jiao, W.; Ban, Y.; Shi, Z.; Jiang, X.; Li, Y.; Yang, W. Gas separation performance of supported carbon molecular sieve membranes based on soluble polybenzimidazole. J. Membr. Sci. 2017, 533, 1–10. [Google Scholar] [CrossRef]
- Giorno, L.; Drioli, E.; Strathmann, H. The principle of gas separation. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Kononova, S.V.; Gubanova, G.N.; Korytkova, E.N.; Sapegin, D.A.; Setnickova, K.; Petrychkovych, R.; Uchytil, P. Polymer Nanocomposite Membranes. Appl. Sci. 2018, 8, 1181. [Google Scholar] [CrossRef] [Green Version]
- Kiyono, M.; Koros, W.J.; Williams, P.J. Correlation between pyrolysis atmosphere and carbon molecular sieve membrane performance properties. Membr. Sci. Technol. 2011, 14, 137–173. [Google Scholar]
- LENNTECH. Membrane Technology. Available online: https://www.lenntech.com/membrane-technology.htm#:~:text=The%20membrane%20separation%20process%20is,suspended%20solids%20and%20other%20substances.&text=Membranes%20occupy%20through%20a%20selective%20separation%20wall (accessed on 21 December 2020).
- Complete Water Solution. The Principle of Reverse Osmosis and Nanofiltration Membrane. Available online: https://complete-water.com/resources/the-principle-of-reverse-osmosis-and-nanofiltration (accessed on 21 December 2020).
- Glover, F.A. Principles of Ultrafiltration and the Concentration and Fractionation of Cow’s Milk. Human Milk Banking. 1984, pp. 1–16. Available online: https://www.nestlenutrition-institute.org/docs/default-source/global-dcoument-library/publications/secured/222783b622c38ac2bde9c8f878b82e26.pdf?sfvrsn=455b1ee3_0#:~:text=The%20principle%20of%20ultrafiltration%20(UF,larger%20molecules%20to%20be%20retained (accessed on 21 December 2020).
- Agboola, O.; Okoli, B.; Sanni, S.E.; Alaba, P.A.; Popoola, P.; Sadiku, E.R.; Mubiayi, P.M.; Akinlabi, E.T.; Makhatha, M.E. Synthesis of activated carbon from olive seeds: Investigating the yield, energy efficiency, and dye removal capacity. SN Appl. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Msaadi, R.; Yilmaz, G.; Allushi, A.; Hamadi, S.; Ammar, S.; Chehimi, M.M.; Yagci, Y. Highly selective copper ion imprinted clay/polymer nanocomposites prepared by visible light initiated radical photo polymerization. Polymer 2019, 11, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, B.; Zhang, X.; Jiang, Z.; Li, Z.; Zhang, Q.; Chen, J. Polymer and polymer-based nanocomposite adsorbents for water treatment. In Polymeric Materials for Clean Water; Das, R., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Bhaumik, M.; Setshedi, K.; Maity, A.; Onyango, M.S. Chromium (VI) removal from water using fixed bed column of polypyrrole/Fe3O4 nanocomposite. Sep. Purif. Technol. 2013, 110, 11–19. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Chen, M.; Gu, H.; Rapole, S.B.; Pallavkar, S.; Ho, T.C.; Hopper, H.; Guo, Z. Magnetic nanocomposites for environmental remediation. Adv. Powder Technol. 2013, 24, 459–467. [Google Scholar] [CrossRef]
- Kotal, M.; Bhowmick, A.K. Polymer nanocomposites from modified clays: Recent advances and challenges. Prog. Polym. Sci. 2015, 51, 127–187. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manage. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, G.R.; Rene, R.M.; Ma Catalina, A.D. Chromium (III) uptake by agro-waste biosorbents: Chemical characterization, sorption-desorption studies, and mechanism. J. Hazard Mater. 2009, 170, 845–854. [Google Scholar] [CrossRef]
- Javadian, H. Application of kinetic, isotherm and thermodynamic models for adsorption of Co (II) ions on polyaniline/polypyrrole copolymer nanofibers from aqueous solution. J. Ind. Eng. Chem. 2014, 20, 4233–4241. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef]
- Zare, E.N.; Lakouraj, M.M.; Ramezani, A. Effective adsorption of heavy metal cations by superparamagnetic poly(aniline- co-m -phenylenediamine)@Fe3O4 nanocomposite. Adv. Polym. Technol. 2015, 34, 1–11. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Najafi, F.; Neshat, A. Poly (amidoamine-co-acrylic acid) copolymer: Synthesis, characterization and dye removal ability. Ind. Crops Prod. 2013, 42, 119–125. [Google Scholar] [CrossRef]
- Shahabuddin, S. Polyaniline Based Nanocomposites as Adsorbents and Photocatalysts in the Removal of Organic Dyes. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2016. [Google Scholar]
- Ansari, R. Polypyrrole conducting electroactive polymers: Synthesis and stability studies. E J. Chem. 2006, 3, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Deng, B.; Chen, H. Removal of aqueous Hg (II) by polyaniline: Sorption characteristics and mechanisms. Environ. Sci. 2009, 43, 5223–5228. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, L.; Duan, W.; Han, L.; Chen, Y. Adsorption of aqueous Cr(VI) by novel fibrous adsorbent with amino and quaternary ammonium groups. Ind. Eng. Chem. Res. 2012, 51, 13655–13662. [Google Scholar] [CrossRef]
- Riahi Samani, M.; Borghei, S.M.; Olad, A.; Chaichi, M.J. Influence of polyaniline synthesis conditions on its capability for removal and recovery of chromium from aqueous solution. Iranian J. Chem. Chem. Eng. 2011, 30, 97–100. [Google Scholar]
- Samani, M.R.; Borghei, S.M. Removal of chromium from aqueous solution using synthesized polyaniline in acetonitrile. Int. J. Mater. Metal. Eng. 2012, 8, 741–744. [Google Scholar]
- Guo, X.; Fei, G.; Su, H.; Zhang, L. High-performance and reproducible polyaniline nanowire/tubes for removal of Cr(VI) in aqueous solution. J. Phys. Chem. C 2011, 115, 1608–1613. [Google Scholar] [CrossRef]
- Ayad, M.; El-Hefnawy, G.; Zaghlol, S. Facile synthesis of polyaniline nanoparticles; its adsorption behavior. Chem. Eng. J. 2013, 217, 460–465. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Zhao, L. Sono-assisted synthesis of nanostructured polyaniline for adsorption of aqueous Cr(VI): Effect of protonic acids. Chem. Eng. J. 2014, 239, 123–131. [Google Scholar] [CrossRef]
- Mahmud, H.N.M.E.; Huq, A.K.O.; Yahya, R. Polymer-based adsorbent for heavy metals removal from aqueous solution. IOP Conf. Series: Mater. Sci. Eng. 2016, 206, 012100. [Google Scholar] [CrossRef]
- Kudoh, Y. Properties of polypyrrole prepared by chemical polymerization using aqueous solution containing Fe2(SO4)3 and anionic surfactant. Synth. Met. 1996, 79, 17–22. [Google Scholar] [CrossRef]
- Deng, S.; Bai, R. Removal of trivalent and hexavalent chromium with aminated polyacrylonitrile fibers: Performance and mechanisms. Water Res. 2004, 38, 2424–2431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, R.; Tong, Y.W. Selective adsorption behaviors of proteins on polypyrrole-based adsorbents. Sep. Purif. Technol. 2006, 52, 161–169. [Google Scholar] [CrossRef]
- Abdi, S.; Nasiri, N.; Mesbahi, A.; Khani, M.H. Investigation of uranium (VI) adsorption by polypyrrole. J. Hazard Mater. 2017, 332, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, M.A.; Khandaker, M.U.; Ladan, M.; Bradley, D.A. Polypyrrole-based nanocomposite adsorbents and its application in removal of radioactive materials. In Polymer-Based Nanocomposites for Energy and Environmental Applications; Woodhead Publishing: Cambridge, UK, 2018; pp. 465–489. [Google Scholar]
- Chávez-Guajardo, A.E.; Medina-Llamas, J.C.; Maqueira, L.; Andrade, C.A.S.; Alves, K.G.B.; de Melo, C.P. Efficient removal of Cr (VI) and Cu (II) ions from aqueous media by use of polypyrrole/maghemite and polyaniline/maghemite magnetic nanocomposites. Chem. Eng. J. 2015, 281, 826–836. [Google Scholar] [CrossRef]
- Kang, H.; Kim, D. Transformation of nanoparticle magnetite prepared in homogeneous aqueous solution. B Korean Chem. Soc. 1998, 19, 408–410. [Google Scholar]
- Muliwa, A.M.; Leswif, T.Y.; Onyango, M.S.; Maity, A. Magnetic adsorption separation (MAS) process: An alternative method of extracting Cr(VI) from aqueous solution using polypyrrole coated Fe3O4 nanocomposites. Sep. Purif. Technol. 2016, 158, 250–258. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Ho, W.H.; Maity, A.; Khenfouch, M.; Srinivasu, V. Removal of hexavalent chromium ions from wastewater using PPY/Fe3O4 magnetic nanocomposite influenced by rotating magnetic field a 2-pole three phase induction motor. J. Phys. Conf. Ser. 2018, 984, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, G.; Tamilarasan, R.; Dharmendirakumar, M. Adsorption, kinetic, equilibrium and thermodynamic studies on the removal of basic dye Rhodamine-B from aqueous solution by the use of natural adsorbent perlite. J. Mater. Environ. Sci. 2012, 3, 157–170. [Google Scholar]
- Saad, M.; Tahir, H. Synthesis of carbon loaded γ-Fe2O3 nanocomposite and their applicability for the selective removal of binary mixture of dyes by ultrasonic adsorption based on response surface methodology. Ultrason. Sonochem. 2017, 36, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, S.K.; Sharma, G.; Julka, J.M.; Kumar, A.; Sharma, S.; Stadler, F.J. Impact of heavy metals and nanoparticles on aquatic biota. Environ. Chem. Lett. 2018, 16, 919–946. [Google Scholar] [CrossRef]
- Achmad, A.; Kassim, J.; Suan, T.K.; Amat, R.C.; Seey, T.L. Equilibrium, kinetic and thermodynamic studies on the adsorption of direct dye onto a novel green adsorbent developed from Uncaria Gambir extract. J. Phys. Sci. 2012, 23, 1–13. [Google Scholar]
- Chagas, N.V.; Meira, J.S.; Anaissi, F.J.; Melquiades, F.L.; Quináia, S.P.; Felsner, M.L.; Justi, K.C. Preparation, characterization of bentonite clay/activated charcoal composites and 23 factorial design application in adsorption studies of methylene blue dye. Rev Virtual Quim. 2013, 6, 1607–1623. [Google Scholar]
- Chang, M.Y.; Juang, R.S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interface Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, E.; Zaharia, C.; Radu, I.C.; Surdu, V.A.; Vasile, B.S.; Damian, C.-M.; Andronescu, E. Novel Nanocomposites Based on Functionalized Magnetic Nanoparticles and Polyacrylamide: Preparation and Complex Characterization. Nanomaterials 2019, 9, 1384. [Google Scholar] [CrossRef] [Green Version]
- Mudhoo, A.; Gautam, R.K.; Ncibi, M.C.; Zhao, F.; Garg, V.K.; Sillanpää, M. Green synthesis, activation and functionalization of adsorbents for dye sequestration. Environ. Chem. Lett. 2019, 17, 157–193. [Google Scholar] [CrossRef]
- Maleki, A.; Hamesadeghi, U.; Daraei, H.; Hayati, B.; Najaf, F.; McKay, G.; Rezaee, R. Amine functionalized multi-walled carbon nanotubes: Single and binary systems for high capacity dye removal. Chem. Eng. J. 2017, 313, 826–835. [Google Scholar] [CrossRef]
- Debnath, S.; Ballav, N.; Maity, A.; Pillay, K. Competitive adsorption of ternary dye mixture using pine cone powder modifed with β-cyclodextrin. J. Mol. Liq. 2017, 225, 679–688. [Google Scholar] [CrossRef]
- Sojoudi, M.; Shariati, S.; Khabazipour, M. Amine functionalized Kit-6 mesoporous magnetite nanocomposite as an efficient adsorbent for removal of Ponceau 4R dye from aqueous solutions. Anal. Bioanal. Chem. Res. 2016, 3, 287–298. [Google Scholar]
- Lau, Y.J.; Khan, F.S.M.; Mubarak, N.M.; Lau, S.Y.; Chua, H.B.; Khalid, M.; Abdullah, E.C. Functionalized carbon nanomaterials for wastewater treatment. In Industrial Applications of Nanomaterials; Micro and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–311. [Google Scholar]
- Adeola, A.O.; Fapohunda, O.; Jimoh, A.T.; Toluwaloju, T.I.; Ige, A.O.; Ogunleye, A.C. Scientific applications and prospects of nanomaterials: A multidiciplinary review. African J. Biotech. 2019, 18, 946–961. [Google Scholar] [CrossRef] [Green Version]
- Homaeigohar, S. The nanosized dye adsorbents for water treatment: Review. Nanomaterials 2020, 10, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tara, N.; Siddiqui, S.L.; Rathi, G.; Chaudhry, S.A.; Inamuddin; Asiri, A.M. Nano-engineered adsorbent for the removal of dyes from water: A Review. Curr. Anal. Chem. 2019, 15, 1–25. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, C.; Xu, W. Magnetic dendritic materials for highly efficient adsorption of dyes and drugs. ACS Appl. Mater. Interfaces 2010, 2, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, X.; He, X.W.; Chen, L.X.; Zhang, Y.K. A self-assembled polydopamine film on the surface of magnetic nanoparticles for specific capture of protein. Nanoscale 2012, 4, 3141–3147. [Google Scholar] [CrossRef]
- Xie, L.J.; Jiang, R.F.; Zhu, F.; Liu, H.; Ouyang, G.F. Application of functionalized magnetic nanoparticles in sample preparation. Anal. Bioanal. Chem. 2014, 406, 377–399. [Google Scholar] [CrossRef]
- Zhang, X.L.; Niu, H.Y.; Pan, Y.Y.; Shi, Y.L.; Cai, Y.Q. Chitosan-coated octadecyl functionalized magnetite nanoparticles: Preparation and application in extraction of trace pollutants from environmental water samples. Anal. Chem. 2010, 82, 2363–2371. [Google Scholar] [CrossRef]
- Atta, A.; Akl, M.C.; Youssef, A.M.; Ibrahim, M.A. Superparamagnetic core-shell polymeric nanocomposites for efficient removal of methylene blue from aqueous solutions. Adsorp. Sci. Technol. 2013, 31, 397–418. [Google Scholar] [CrossRef]
- Dai, R.; Zhang, Y.; Shi, Z.-Q.; Yang, F.; Zhao, C.-H. A facile approach towards amino-coated ferroferric oxide nanoparticles for environmental pollutant removal. J. Colloid Interface Sci. 2018, 513, 647–657. [Google Scholar] [CrossRef]
- Muntean, S.G.; Nistor, M.A.; Muntean, E.; Todea, A.; Ianos, R.; Pacurariu, C. Removal of Colored Organic Pollutants from Wastewaters by Magnetite/Carbon Nanocomposites: Single and Binary Systems. J. Chem. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Asthana, A.; Chakraborty, R.; Jain, B.; Singh, A.K.; Carabineiro, S.A.C.; Susan, M.A.B.H. Cationic dye removal using novel magnetic/activated charcoal/βCyclodextrin/alginate polymer nanocomposite. Nanomaterials 2020, 10, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanisamy, K.; Jeyaseelan, A.; Murugesan, K.; Babu, P.S.; Karthikeyan, S. Environmental applications of chitosan and cellulosic biopolymers: A comprehensive outlook. Bioresour. Technol. 2017, 242, 295–302. [Google Scholar]
- Sadeghi-Kiakhani, M.; Arami, M.; Gharanjig, K. Dye removal from colored-textile wastewater using chitosan-PPI dendrimer hybrid as a biopolymer: Optimization, kinetic, and isotherm studies. J. Appl. Polym. Sci. 2013, 127, 2607–2619. [Google Scholar] [CrossRef]
- Liu, S.; Chen, D.; Zheng, J.; Zeng, L.; Jiang, J.; Jiang, R.; Zhu, F.; Shen, Y.; Wu, D.; Ouyang, G. The sensitive and selective adsorption of aromatic compounds with highly crosslinked polymer nanoparticles. Nanoscale 2015, 7, 16943–16951. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.; Haratipour, P.; Ahmadi, M.; Zare-Dorabei, R.; Mahmoodi, A. Efficient removal of some anionic dyes from aqueous solution using a polymer-coated magnetic nano-adsorbent. J. Water Supply Res. Technol. Aqua 2017, 66, 239–248. [Google Scholar] [CrossRef]
- Saad, M.; Tahir, H.; Khan, J.; Hameed, U.; Saud, A. Synthesis of polyaniline nanoparticles and their application for the removal of Crystal Violet dye by ultrasonic adsorption process based on response surface methodology. Ultrason. Sonochem. 2017, 34, 600–608. [Google Scholar] [CrossRef]
- Nakhjiri, M.T.; Marandi, G.B.; Kurdtabar, M. Poly(AA-co-VPA) hydrogel cross-linked with N-maleyl chitosan as dye adsorbent: Isotherms, kinetics and thermodynamic investigation. Int. J. Biol. Macromol. 2018, 117, 152–166. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Ghfar, A.A.; Al-Muhtaseb, A.H.; Ahamad, T.; Sharma, N.; Stadler, F.J. Carboxymethyl cellulose structured nano-adsorbent for removal of methyl violet from aqueous solution: Isotherm and kinetic analyses. Cellulose 2020, 27, 3677–3691. [Google Scholar] [CrossRef]
- Da Silver, R.C.; de Aguiar, S.B.; da Cunha, P.L.R.; de Paula, R.C.M.; Feitosa, J.P.A. Effect of microwave on the synthesis of polyacrylamide-g-chitosan gel for azo dye removal. React. Funct. Polym. 2020, 148, 104–491. [Google Scholar] [CrossRef]
- Ugraskan, V.; Torman, A.; Yoruc, A.B.H. Chitosan-Based Adsorbents for Wastewater Treatment; Naser, A., Ed.; Materials Research Forum LLC: Millersville, PA, USA, 2018; pp. 2–17. [Google Scholar]
- Vafakish, B.; Wilson, L.D. Surface-Modified chitosan: An adsorption study of a “Tweezer-Like” biopolymer with fluorescein. Surfaces 2019, 2, 468–484. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-an overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef] [PubMed]
- Sadegh, H.; Ali, G.A.M.; Makhlouf, A.S.H.; Chong, K.F.; Alharbi, N.S.; Agarwal, S.; Gopta, V.K. MWCNTs-Fe3O4 nanocomposite for Hg(II) high adsorption efficiency. J. Mol. Liq. 2018, 258, 345–353. [Google Scholar] [CrossRef]
- Sheibani, M.; Ghaedi, M.; Marahel, F.; Ansari, A. Congo red removal using oxidized multiwalled carbon nanotubes: Kinetic and isotherm study. Desalin. Water Treat. 2015, 53, 844–852. [Google Scholar] [CrossRef]
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of metal-organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal. Chem. Eng. J. 2017, 15, 1145–1158. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; Heer, W.A.D. Carbon nanotubes. Science 2002, 297, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-Y.; Xu, X. Wrapping carbon nanotubes with poly (sodium 4- styrenesulfonate) for enhanced adsorption of methylene blue and its mechanism. Chem. Eng. J. 2014, 256, 85–92. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Hosseinzadeh, H.; Pashaei, S.; Khodaparast, Z. Synthesis of magnetic functionalized MWCNT nanocomposite through surface RAFT co-polymerization of acrylic acid and N-isopropyl acrylamide for removal of cationic dyes from aqueous solutions. Ecotox. Environ. Saf. 2018, 161, 34–44. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, M.; Yang, M.; Wang, W.; Zhang, S.; Han, R. Adsorption of light green anionic dye from solution using polyethyleneimine-modified carbon nanotubes in batch mod. Desalination 2019, 138, 368–378. [Google Scholar]
- Yang, G.; Huang, Q.; Gan, D.; Huang, H.; Chen, J.; Deng, F.; Liu, M.; Wen, Y.; Zhang, X.; Wei, Y. Biomimetic functionalization of carbon nanotubes with poly(ionic liquids) for highly efficient adsorption of organic dyes. J. Mol. Liq. 2019, 296, 112059. [Google Scholar] [CrossRef]
- Gan, D.; Dou, J.; Huang, Q.; Huang, H.; Chen, J.; Liu, M.; Qi, H.; Yang, Z.; Zhang, X.; Wei, Y. Carbon nanotubes-based polymer nanocomposites: Bio-mimic preparation and methylene blue adsorption. J. Environ. Chem. Eng. 2020, 8, 103525. [Google Scholar] [CrossRef]
- Mohammad-Salim, H.A. Oxygen functionalized and pristine carbon nanotubes efficiency for adsorption of methyl orange dye. Inter. Res. J. Pure. Appl. Chem. 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Niranjana, K.; Anthony, G.J.M.; Raja, S. A study on the adsorption of gases in the thin film nanocomposite. Mater. Today Proceed. 2019, 8, 79–84. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Carbon dioxide adsorption in graphene sheets. AIP Adv. 2011, 1, 1–7. [Google Scholar] [CrossRef]
- Ghazali, A.A.; Rahman, S.A.; Samah, R.A. Potential of adsorbents from agricultural wastes as alternative fillers in mixed matrix membrane for gas separation: A review. Green Process Synth. 2020, 9, 219–229. [Google Scholar] [CrossRef]
- Carta, M. Gas separation. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Heidelberg, Germany, 2015; pp. 852–855. [Google Scholar]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Wang, B.; Xie, L.-H.; Wang, X.; Liu, X.-M.; Li, J.; Li, J.-R. Applications of metal-organic frameworks for green energy and environment: New advances in adsorptive gas separation, storage and removal. Green Energy Environ. 2018, 3, 191–228. [Google Scholar] [CrossRef]
- Hu, T.-L.; Wang, H.; Li, B.; Krishna, R.; Wu, H.; Zhou, W.; Zhao, Y.; Han, Y.; Wang, X.; Zhu, W.; et al. Microporous metal-organic framework with dual functionalities for highly efficient removal of acetylene from ethylene/acetylene mixtures. Nat. Comms. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.-G.; Lin, R.-B.; Chen, B. A microporous metal–organic framework for selective C2H2 and CO2 separation. J. Solid State Chem. 2017, 252, 138–141. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, D.; Wu, J.; Xiao, J.; Xi, H.; Xia, Q.; Li, Z. A new MOF-505@ GO composite with high selectivity for CO2/CH4 and CO2/N2 separation. Chem. Eng. J. 2017, 308, 1065–1072. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Z.; Wu, H.; Lv, D.; Shi, R.; Xia, Q.; Zhou, J.; Li, Z. An ethane-trapping MOF PCN-250 for highly selective adsorption of ethane over ethylene. Chem. Eng. Sci. 2018, 175, 110–117. [Google Scholar] [CrossRef]
- Karimi, S.; Yaraki, M.T.; Karri, R.R. A comprehensive review of the adsorption mechanisms and factors influencing the adsorption process from the perspective of bioethanol dehydration. Renew Sust. Energ. Rev. 2019, 107, 535–553. [Google Scholar] [CrossRef]
- Azhar, A.; Farshi, F. Eco-friendly biopolymer/clay/conducting polymer nanocomposite: Characterization and its application in reactive dye removal. Fibers Polym. 2014, 15, 1321–1329. [Google Scholar]
- Wang, M.; Gu, Q.; Luo, Y.; Bukhvalov, D.; Ma, X.; Zhu, L.; Li, G.; Luo, Z. Understanding mechanism of adsorption in the decolorization of aqueous methyl violet (6B) solution by okra polysaccharides: Experiment and theory. ACS Omega 2019, 4, 17880–17889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noonan, G.O.; Whelton, A.J.; Carlander, D.; Duncan, T.V. Measurement methods to evaluate engineered nanomaterial release from food contact materials. Compr. Rev. Food Sci. Food Saf. 2014, 13, 679–692. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef] [Green Version]
- Norman, N.L.; Fane, A.G.; Winston Ho, W.S.; Matsuura, T. Advanced Membrane Technology and Applications; John Wiley & Sons Ltd.: Chichester, UK, 2008. [Google Scholar]
- Dudziak, M.; Bodzek, M. Factors driving rejection of micropollutants (xenoestrogens and phytoestrogens) during reverse osmosis/nanofiltration treatment. Archit. Civ. Eng. Environ. 2010, 1, 95–102. [Google Scholar]
- Duncan, T.V.; Pillai, K. Release of Engineered Nanomaterials from Polymer Nanocomposites: Diffusion, Dissolution, and Desorption. Appl. Mater. Interface 2015, 7, 2–19. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric system. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Agboola, O.; Sadiku, E.R.; Mokrani, T. Nanomembrane materials based on polymer blends. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Elsevier: Amsterdam, The Netherlands, 2016; pp. 101–123. [Google Scholar]
- Li, Y.; Tao, P.; Viswanath, A.; Benicewicz, B.C.; Schadler, L.S. Bimodal surface ligand engineering: The key to tunable nanocomposites. Langmuir 2012, 29, 1211–1220. [Google Scholar] [CrossRef]
- Gusev, A.A. Micromechanical mechanism of reinforcement and losses in filled rubbers. Macromolecules 2006, 39, 5960–5962. [Google Scholar] [CrossRef]
- Ounaies, Z.; Park, C.; Wise, K.; Siochi, E.; Harrison, J. Electrical properties of single wall carbon nanotube reinforced polyimide composites. Compos. Sci. Technol. 2003, 63, 1637–1646. [Google Scholar] [CrossRef]
- Forrest, J.A.; Dalnoki-Veress, K. The glass transition in thin polymer films. Adv. Colloid Interface Sci. 2001, 94, 167–195. [Google Scholar] [CrossRef]
- Senses, E.; Faraone, A.; Akcora, P. Microscopic chain motion in polymer nanocomposites with dynamically asymmetric interphases. Sci. Rep. 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittigstein, P.; Priestley, R.D.; Broadbelt, L.J.; Torkelson, J.M. Model polymer nanocomposites provide an understanding of confinement effects in real nanocomposites. Nat. Mat. 2007, 6, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Obukhov, S.; Chen, J.-T.; Huh, J.; Hwang, Y.; Mok, S.; Dobriyal, P.; Thiyagarajan, P. Enhanced mobility of confined polymers. Nat. Mat. 2007, 6, 961–965. [Google Scholar] [CrossRef]
- Chandran, S.; Basu, J.K.; Mukhopadhyay, M.K. Variation in glass transition temperature of polymer nanocomposite films driven by morphological transitions. J. Chem. Phys. 2013, 138, 1–6. [Google Scholar] [CrossRef]
- Berens, A.R.; Hopfenberg, H.B. Diffusion of organic vapors at low concentrations in glassy PVC, polystyrene and PMMA. J. Membr. Sci. 1982, 10, 283–303. [Google Scholar] [CrossRef]
- Hashem, E.A. Nanotechnology in water treatment, case study. Egypt J. Econ. Dev. Stud. 2014, 2, 243–259. [Google Scholar] [CrossRef]
- Frandsen, M.V.; de Champvermeil, J.L.M.; Pascal, J.M. Gravity-Driven Water Purification System and Method for Manufacturing a Flexible, Collapsible Container. UK Patent 2531177: WO2016/162035 En, 13 October 2016. [Google Scholar]
- Rodrigues, D.F.; Advincula, R.C.; Claydon, F.; Santos, C.M.; Tria, M.C.R. Nanocomposite Polymer-Carbon Based Nanomaterial Filter for the Simultaneous Removal of Bacteria and Heavy Metals. U.S. Patent WO2013039895A1, 21 March 2013. [Google Scholar]
- Lind, M.A.; Fink, S.; Sasaki, K.; Lin, J. Nanocomposite Membranes. U.S. Patents WO 2014/159352A1, 11 March 2014. [Google Scholar]
- Lahalih, S.M. Nanocomposite Mixed-Matrix Membrane. Justia Patents 9,073,014, 1 October 2014. [Google Scholar]
- Bhattacharyya, D.; Smuleac, V.; Varma, R.S.; Sikdar, S.K. Green Synthesis Nanocomposite Membranes. U.S. Patent US 10,245,558 B2, 2 April 2019. [Google Scholar]
- Kang, Y.; Char, K.; Kang, S. Silver Nanoparticle/Polymer Nanocomposite Membranes for Olefin/Paraffin Separation and Method of Preparing the Same. U.S. Patent US20,070,012,189A1, 17 February 2007. [Google Scholar]
- Fritsch, D.; Merten, P. Composite Membrane, in Particular for Gas Separation. European Patent EP2,397,218A1, 21 December 2011. [Google Scholar]
- Vijayendran, B.R.; Lalgudi, R.S. Polymer Nanocomposites for Gas Separation. U.S. Patent US 9.126,137 B1, 8 September 2015. [Google Scholar]
- Paul, P.; Nataraj, S.K.; Kumar, A.; Prajapati, P.K. Sustainable Biomaterial Nanocomposites for Water Treatment and Process for Preparation Thereof. International Patent WO2,017,002,137A1, 5 January 2017. [Google Scholar]
- Alqadami, A.A.; Khan, M.A.; Alothman, Z.A.; Alsohaimi, I.H.; Siddiqui, M.R.; Ghfar, A.A. Magnetic Polymer Nanocomposite for Removal of Divalent Heavy Metal Ions from Water. U.S. Patent US10,245,576B1, 2 April 2019. [Google Scholar]
- Long, J.R.; Herm, Z.R.; Swisher, J.A.; Smit, B.; Krishna, R.; Bloch, E.; Murray, L. Metal-Organic Framework Adsorbents for Composite Gas Separation. U.S. Patent US20,140,061,540A1, 6 March 2014. [Google Scholar]
- Allendorf, M.D.; Greathouse, J.A.; Chad, S. Metal-Organic Frameworks for Adsorption and Separation of Noble Gases. Justia Patents 9,662,632, 18 March 2015. [Google Scholar]
- Schroder, M.; Yang, S. Metal-Organic Frameworks (Mof) for Gas Capture. European Patent EP2,846,896B1, 24 April 2019. [Google Scholar]
- Abu-Dief, A.M.; Alzahrani, S.O. Sustainability nanocomposite for water treatment. Int. J. Org. Inorg. Chem. 2020, 2, 1–12. [Google Scholar]
- Babatunde, D.E.; Denwigwe, I.H.; Babatunde, O.M.; Gbadamosi, S.L.; Babalola, I.P.; Agboola, O. Environmental and societal impact of nanotechnology. IEEE Access 2019, 8, 4640–4667. [Google Scholar] [CrossRef]
- Beyene, H.D.; Ambaye, T.G. Application of Sustainable Nanocomposites for Water Purification Process. In Sustainable Polymer Composites and Nanocomposites; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 387–412. [Google Scholar]







| Nanomaterials | Properties | References |
|---|---|---|
| Nanoparticles | -Possess huge surface area to volume ratio -Possess high percentage of atoms/molecules associated with surfaces -Have exceptional chemical and physical properties. -Possess unique optical properties that depend on the size, which conveys diverse colours as a result of absorption in the noticeable section. -Possess high reactivity and toughness properties that depend on their distinctive structure, size, and shape. -Possess strong particle mobility -Possess strong surface energy and colloid stabilisation via the provision of barricade to close approach of two particles. -Possess same size scale as many biological molecules. | [2,3,12,13,14,15] |
| Carbon nanotubes | -Possess high thermal conductivity -Possess a remarkable electrical conductivity -Possess a remarkable mechanical property -Possess a large length-to-diameter ratio (aspect ratio) of higher than 1000 -The images of the actual space examination of nanotube have revealed a series of inter-stratum spacing -Single walled nanotube generally comprises of only 10 atoms near the circumference and the thickness of the tube is only one-atom thick | [3,16,17,18] |
| Nanosheets | -Exhibits high surface area that makes them advantageous for the fabrication of excellent reinforced polymeric composites -Their surfaces contain a large quantity of active oxygen-containing groups -Possess excellent mechanical and thermal conductivity properties -Possess excellent catalytic activities such as photo-/thermo-catalytic activity -Possess excellent thermal and electrical conductivity | [5,6,19,20,21] |
| Nanofibers | -Nanofibers are very small in size, which accords them outstanding physical and chemical properties -Possess huge surface area, high aspect ratio, and superior surface properties, which is responsible for their suitability for other technologies that need a smaller environment for chemical reaction to take place -Possess high pore volume and tight pore size that accords their suitability for an extensive range of filtration applications -Possess extreme adsorption capacity that has the capacity to improve many applications | [4,22,23,24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agboola, O.; Fayomi, O.S.I.; Ayodeji, A.; Ayeni, A.O.; Alagbe, E.E.; Sanni, S.E.; Okoro, E.E.; Moropeng, L.; Sadiku, R.; Kupolati, K.W.; et al. A Review on Polymer Nanocomposites and Their Effective Applications in Membranes and Adsorbents for Water Treatment and Gas Separation. Membranes 2021, 11, 139. https://doi.org/10.3390/membranes11020139
Agboola O, Fayomi OSI, Ayodeji A, Ayeni AO, Alagbe EE, Sanni SE, Okoro EE, Moropeng L, Sadiku R, Kupolati KW, et al. A Review on Polymer Nanocomposites and Their Effective Applications in Membranes and Adsorbents for Water Treatment and Gas Separation. Membranes. 2021; 11(2):139. https://doi.org/10.3390/membranes11020139
Chicago/Turabian StyleAgboola, Oluranti, Ojo Sunday Isaac Fayomi, Ayoola Ayodeji, Augustine Omoniyi Ayeni, Edith E. Alagbe, Samuel E. Sanni, Emmanuel E. Okoro, Lucey Moropeng, Rotimi Sadiku, Kehinde Williams Kupolati, and et al. 2021. "A Review on Polymer Nanocomposites and Their Effective Applications in Membranes and Adsorbents for Water Treatment and Gas Separation" Membranes 11, no. 2: 139. https://doi.org/10.3390/membranes11020139