Separation Iron(III)-Manganese(II) via Supported Liquid Membrane Technology in the Treatment of Spent Alkaline Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Methods
2.2.1. Liquid-Liquid Extraction Experiments
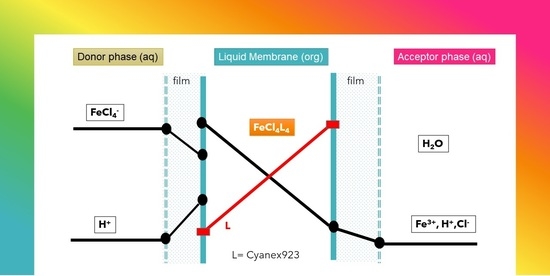
2.2.2. SLM Experiments
3. Results
3.1. Iron(III) Liquid-Liquid Extraction Equilibrium
3.2. Iron(III) Transport through the Supported Liquid Membrane
3.2.1. Influence of the Stirring Speed Applied on the Feed Phase on Iron(III) Transport
3.2.2. Influence of the Stirring Speed Applied on the Receiving Phase on Iron(III) Transport
3.2.3. Influence of the HCl Concentration in the Feed Phase on Iron(III) Transport
3.2.4. Influence of Cyanex 923 Concentration on Iron(III) Transport
3.2.5. Influence of Manganese(II) Concentration in the Feed Phase on Iron(III) Transport
3.2.6. Estimation of Diffusional Parameters and Evaluation of Mass Transfer Resistances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caravaca, C.; Cobo, A.; Alguacil, F.J. Considerations about the recycling of EAF flue dusts as source for the recovery of valuable metals by hydrometallurgical processes. Resour. Conserv. Recycl. 1994, 10, 35–41. [Google Scholar] [CrossRef]
- Alguacil, F.J.; de Goicoechea, N.; Dañobeitia, I.; Cobo, A.; Caravaca, C.; García, F. Procedimiento para la Obtención de un Óxido de Cinc de Alta Pureza Mediante Lixiviación de Óxidos Waelz con Disoluciones de Carbonato Amónico. Spanish Patent 9,500,605, 28 March 1995. [Google Scholar]
- Ruiz, O.; Clemente, C.; Alonso, M.; Alguacil, F.J. Recycling of an electric arc furnace flue dust to obtain high grade ZnO. J. Hazard. Mat. 2007, 141, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Cebriano, T.; García-Díaz, I.; López Fernández, A.; Fernández, P.; López, F.A. Synthesis and characterization of ZnO micro- and nanostructures grown from recovered ZnO from spent alkaline batteries. J. Environ. Chem. Eng. 2017, 5, 2903–2911. [Google Scholar] [CrossRef]
- López, F.A.; Cebriano, T.; García-Díaz, I.; Fernández, P.; Rodríguez, O.; López Fernández, A. Synthesis and microstructural properties of zinc oxide nanoparticles prepared by selective leaching of zinc from spent alkaline batteries using ammoniacal ammonium carbonate. J. Clean. Prod. 2017, 148, 795–803. [Google Scholar] [CrossRef]
- Rzelewska-Piekut, M.; Regel-Rosocka, M. Liquid membranes for separation of metal ions from wastewaters. Phys. Sci. Rev. 2021, in press. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Supported ionic liquid and polymer inclusion membranes for metal separation. Sep. Purif. Rev. 2021, in press. [Google Scholar] [CrossRef]
- Yesil, H.; Molaey, R.; Calli, B.; Tugtas, A.E. Removal and recovery of heavy metals from sewage sludge via three-stage integrated process. Chemosphere 2021, 280, 130650. [Google Scholar] [CrossRef] [PubMed]
- Othmen, K.; Ncib, S.; Barhoumi, A.; Dammak, L.; Bouguerra, W. Recovery of nickel ions by supported liquid membrane (Slm) using d2ehpa as carrier. Desalin. Water Treat. 2021, 225, 422–429. [Google Scholar] [CrossRef]
- Lee, L.Y.; Morad, N.; Ismail, N.; Rafatullah, M. Selective separation of cadmium (II), copper (II) and nickel (II) ions from electroplating wastewater using dual flat sheet supported liquid membrane. Desalin. Water Treat. 2021, 224, 291–301. [Google Scholar] [CrossRef]
- Patel, M.; Karamalidis, A.K. Germanium: A review of its US demand, uses, resources, chemistry, and separation technologies. Sep. Purif. Technol. 2021, 275, 118981. [Google Scholar] [CrossRef]
- Patil, A.B.; Struis, R.P.W.J.; Testino, A.; Ludwig, C. Extraction of Rare Earth Metals: The New Thermodynamic Considerations Toward Process Hydrometallurgy. Miner. Met. Mater. Ser. 2021, 187–194. [Google Scholar] [CrossRef]
- Mahanty, B.; Karak, A.; Mohapatra, P.K.; Egberink, R.J.M.; Valsala, T.P.; Sathe, D.B.; Bhatt, R.B.; Huskens, J.; Verboom, W. Carrier mediated transport of actinides using hexa-n-hexylnitrilotriacetamide (HHNTA). Chem. Eng. Process. Process Intensif. 2021, 161, 108323. [Google Scholar] [CrossRef]
- Rout, P.C.; Sarangi, K. A systematic study on extraction and separation of scandium using phosphinic acid by both solvent extraction and hollow fibre membrane. Miner. Process. Extr. Metall. Trans. Inst. Min. Metall. 2021, in press. [Google Scholar] [CrossRef]
- Kumar, R.; Kandwal, P. Mathematical Modeling of facilitated transport of Eu (III) ion by CMPO in modified diluents as extractant. J. Phys. Conf. Ser. 2021, 1849, 01200. [Google Scholar] [CrossRef]
- Ali, N.; Shah, S.; Khan, A.; Khan, S.B.; Kamal, T.; Asiri, A.M. Selective separation of tungsten from the model and industrial effluents through supported liquid membrane. Chem. Pap. 2021, 75, 553–563. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Alguacil, F.J. Recent trends in metals extraction. Rev. Metal. 2013, 49, 292–316. [Google Scholar] [CrossRef] [Green Version]
- Alguacil, F.J.; Martínez, S. Permeation of iron (III) by an immobilised liquid membrane using Cyanex 923 as mobile carrier. J. Membr. Sci. 2000, 176, 249–255. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Alonso, M. Iron (III) transport using a supported liquid membrane containing Cyanex 921. Hydrometallurgy 2000, 58, 81–88. [Google Scholar] [CrossRef]
- Bohrer, M.P. Diffusional boundary layer resistance for membrane transport. Ind. Eng. Chem. Fundam. 1983, 22, 72–78. [Google Scholar] [CrossRef]
- Pavon, S.; Fortuny, A.; Coll, M.T.; Bertaub, M.; Sastre, A.M. Permeability dependencies on the carrier concentration and membrane viscosity for Y (III) and Eu (III) transport by using liquid membranes. Sep. Purif. Technol. 2020, 239, 116573. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López, F.A. The extraction of mineral acids by the phosphine oxide Cyanex 923. Hydrometallurgy 1996, 42, 245–255. [Google Scholar] [CrossRef]
- De Gyves, J.; De San Miguel, E.R. Metal ion separations by supported liquid membrane. Ind. Eng. Chem. Res. 1999, 38, 2182–2202. [Google Scholar] [CrossRef]
- Sastre, A.M.; Alguacil, F.J.; Alonso, M.; Lopez, F.; Lopez-Delgado, A. On cadmium (II) membrane-based extraction using Cyanex 923 as carrier. Solvent Extr. Ion Exch. 2008, 26, 192–207. [Google Scholar] [CrossRef]
- Huang, T.C.; Juang, R.S. Rate and mechanism of divalent metal transport through supported liquid membrane containing di (2-ethylhexyl) phosphoric acid as a mobile carrier. J. Chem. Technol. Biotechnol. 1988, 42, 3–17. [Google Scholar] [CrossRef]

| Stirring Speed, min−1 | KFe 103, cm/s | % Fe Recovery a |
|---|---|---|
| 500 | 0.7 | 99 |
| 1000 | 1.1 | 99 |
| 1250 | 1.4 | 99 |
| 1500 | 1.5 | 99 |
| [HCl], M | KFe·10−3, cm/s | % Fe Recovery a |
|---|---|---|
| 1 | 0.08 | 99 |
| 1.5 | 0.56 | 99 |
| 2 | 1.4 | 99 |
| 4 | 3.4 | 72 |
| 6 | 3.1 | 68 |
| 8 | 2.3 | 65 |
| [Cyanex 923], % v/v | KFe·10−3, cm/s | % Fe Recovery a |
|---|---|---|
| 1.3 | 1.0 | 90 |
| 2.5 | 2.3 | 99 |
| 5 | 2.5 | 98 |
| 10 | 3.4 | 72 |
| 20 | 2.9 | 71 |
| 30 | 2.3 | 72 |
| 40 | 2.1 | 72 |
| Experimental Condition | a R, s/cm | b Rf, s/cm | %Rf0 | %Rm0 |
|---|---|---|---|---|
| 1–10% v/v Cyanex 923 | 1000–294 | 361 | 36–100 | 64–0 |
| 10–40% v/v Cyanex 923 | 294–476 | 361 | 100–76 | 0–24 |
| 1–4 M HCl | 12,195–294 | 361 | 3–100 | 97–0 |
| 4–8 M HCl | 294–435 | 361 | 100–83 | 0–17 |
| 0–0.17 g/L Mn(II) | 294 | 361 | 100 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alguacil, F.J.; Lopez, F.A. Separation Iron(III)-Manganese(II) via Supported Liquid Membrane Technology in the Treatment of Spent Alkaline Batteries. Membranes 2021, 11, 991. https://doi.org/10.3390/membranes11120991
Alguacil FJ, Lopez FA. Separation Iron(III)-Manganese(II) via Supported Liquid Membrane Technology in the Treatment of Spent Alkaline Batteries. Membranes. 2021; 11(12):991. https://doi.org/10.3390/membranes11120991
Chicago/Turabian StyleAlguacil, Francisco J., and Félix A. Lopez. 2021. "Separation Iron(III)-Manganese(II) via Supported Liquid Membrane Technology in the Treatment of Spent Alkaline Batteries" Membranes 11, no. 12: 991. https://doi.org/10.3390/membranes11120991