Porous Gelatin Membranes Obtained from Pickering Emulsions Stabilized with h-BNNS: Application for Polyelectrolyte-Enhanced Ultrafiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Exfoliated H-BNNS
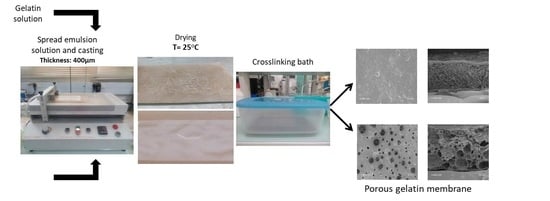
2.3. Preparation of Membranes
2.4. Membrane Characterization
2.4.1. Membrane Morphology
2.4.2. Water Contact Angle
2.4.3. Swelling Ratio
2.4.4. Pore Size Determination
2.5. Membrane Performances
2.5.1. Filtration Tests
2.5.2. Polystyrene Latex Particle Rejection Test
2.5.3. Complexation–Ultrafiltration Procedure
3. Results
3.1. Gelatin Membrane Fabrication and Characterization
3.2. WCA Measurements
3.3. Swelling Ratio
3.4. Pore Size Determination
3.5. Membrane Performances
3.5.1. Filtration
3.5.2. Polystyrene Latex Particle Rejection Test
3.5.3. Complexation–Ultrafiltration Procedure
Ultrafiltration of An Iron Ion Solution
Ultrafiltration of an Iron Ion Solution in the Presence of PAA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNP | silver nanoparticles |
| BSA | bovine serum albumin |
| CNT | carbon nanotubes |
| GO | graphene oxide |
| GTA | glutaraldehyde |
| h-BNNS | Hexagonal boron nitride nanosheets |
| Jpw | flux of pure water |
| Jv | aqueous iron solution permeate |
| Lp | pure water permeability |
| MW | molecular weight |
| NIPS | non-solvent induced phase separation |
| PAA | poly (acrylic acid) |
| PAN | Polyacrylonitrile |
| PEG | polyethylene glycol |
| PEUF | polyelectrolyte-enhanced ultrafiltration |
| PLA | Poly (L-lactic acid) |
| PSU | polysulphone |
| RFe | iron ions rejection percentage |
| SEM | Scanning Electron Microscopy |
| SR | swelling ratio |
| TIPS | thermally induced phase separation |
| WCA | Water Contact Angle |
References
- Geise, G.M.; Lee, H.S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Zaviska, F.; Drogui, P.; Grasmick, A.; Azais, A.; Héran, M. Nanofiltration membrane bioreactor for removing pharmaceutical compounds. J. Memb. Sci. 2013, 429, 121–129. [Google Scholar] [CrossRef]
- Xiao, W.; Zhao, L.; Gong, Y.; Liu, J.; Yan, C. Preparation and performance of poly(vinyl alcohol) porous separator for lithium-ion batteries. J. Memb. Sci. 2015, 487, 221–228. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [Green Version]
- Takht Ravanchi, M.; Kaghazchi, T.; Kargari, A. Application of membrane separation processes in petrochemical industry: A review. Desalination 2009, 235, 199–244. [Google Scholar] [CrossRef]
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Ueno, M.; Watanabe, Y.; Kouya, T.; Taniguchi, M.; Lloyd, D.R. Poly(L-lactic acid) microfiltration membrane formation via thermally induced phase separation with drying. J. Chem. Eng. Jpn. 2011, 44, 467–475. [Google Scholar] [CrossRef]
- Shen, P.; Moriya, A.; Rajabzadeh, S.; Maruyama, T.; Matsuyama, H. Improvement of the antifouling properties of poly (lactic acid) hollow fiber membranes with poly (lactic acid)–polyethylene glycol–poly (lactic acid) copolymers. Desalination 2013, 325, 37–39. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, Q. Gas permeability of a novel cellulose membrane. J. Memb. Sci. 2002, 204, 185–194. [Google Scholar] [CrossRef]
- Tran, T.N.; Athanassiou, A.; Basit, A.; Bayer, I.S. Starch-based bio-elastomers functionalized with red beetroot natural antioxidant. Food Chem. 2017, 216, 324–333. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Guenoun, P.; Garate, H.; Deratani, A.; Quemener, D.; Pochat-Bohatier, C.; Bouyer, D. Fabrication of novel porous membrane from biobased water-soluble polymer (hydroxypropylcellulose). J. Memb. Sci. 2017, 526, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Biscarat, J.; Charmette, C.; Sanchez, J.; Pochat-Bohatier, C. Preparation of dense gelatin membranes by combining temperature induced gelation and dry-casting. J. Memb. Sci. 2015. [Google Scholar] [CrossRef]
- Biscarat, J.; Charmette, C.; Sanchez, J.; Pochat-Bohatier, C. Gas permeability properties of gelatin/polyetheramine blend membranes made without organic solvent. Sep. Purif. Technol. 2015, 142, 33–39. [Google Scholar] [CrossRef]
- Biscarat, J.; Bechelany, M.; Pochat-Bohatier, C.; Miele, P. Graphene-like BN/gelatin nanobiocomposites for gas barrier applications. Nanoscale 2014, 7, 613. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- M’barki, O.; Hanafia, A.; Bouyer, D.; Faur, C.; Sescousse, R.; Delabre, U.; Blot, C.; Guenoun, P.; Deratani, A.; Quemener, D.; et al. Greener method to prepare porous polymer membranes by combining thermally induced phase separation and crosslinking of poly(vinyl alcohol) in water. J. Memb. Sci. 2014, 458, 225–235. [Google Scholar] [CrossRef]
- Ziminska, M.; Dunne, N.; Hamilton, A.R. Porous Materials with Tunable Structure and Mechanical Properties via Templated Layer-by-Layer Assembly. ACS Appl. Mater. Interfaces 2016, 8, 21968–21973. [Google Scholar] [CrossRef] [Green Version]
- Aram, E.; Mehdipour-Ataei, S. A review on the micro- and nanoporous polymeric foams: Preparation and properties. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 358–375. [Google Scholar] [CrossRef]
- Parekh, S.A.; David, R.N.; Bannuru, K.K.R.; Krishnaswamy, L.; Baji, A. Electrospun silver coated polyacrylonitrile membranes for water filtration applications. Membranes 2018, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Manawi, Y.M.; Wang, K.; Kochkodan, V.; Johnson, D.J.; Atieh, M.A.; Khraisheh, M.K. Engineering the surface and mechanical properties of water desalination membranes using ultralong carbon nanotubes. Membranes 2018, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Wu, T.; Xu, X.; Zhang, L.; Wu, F. Factors that affect the stability, type and morphology of Pickering emulsion stabilized by silver nanoparticles/graphene oxide nanocomposites. Mater. Res. Bull. 2014, 60, 118–129. [Google Scholar] [CrossRef]
- Yang, Y.; Ning, Y.; Wang, C.; Tong, Z. Capsule clusters fabricated by polymerization based on capsule-in-water-in- oil Pickering emulsions. Polym. Chem. 2013, 4, 5407–5415. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, X.; Huang, Y.; Wang, H.; Du, Q. Interconnected Porous Polymers with Tunable Pore Throat Size Prepared via Pickering High Internal Phase Emulsions. Langmuir 2016, 32, 38–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Song, X.; Du, Q. Fabrication of Two Kinds of Polymer Microspheres Stabilized by Modified Titania during Pickering Emulsion Polymerization. Macromol. Chem. Phys. 2010, 211, 2517–2529. [Google Scholar] [CrossRef]
- Li, X.; Sun, G.; Li, Y.; Yu, J.C.; Wu, J.; Ma, G.-H.; Ngai, T. Porous TiO2 Materials through Pickering High-Internal Phase Emulsion Templating. Langmuir 2014, 30, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Neirinck, B.; Fransaer, J.; Van der Biest, O.; Vleugels, J. Production of Porous Materials Through Consolidation of Pickering Emulsions. Adv. Eng. Mater. 2007, 9, 57–59. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Yang, H.; Petit, C.; Lee, W. Magnetically controllable Pickering emulsion prepared by a reduced graphene oxide-iron oxide composite. J. Colloid Interface Sci. 2015, 438, 296–305. [Google Scholar] [CrossRef]
- Tang, M.; Wang, X.; Wu, F.; Liu, Y.; Zhang, S.; Pang, X.; Li, X.; Qiu, H. Au nanoparticle/graphene oxide hybrids as stabilizers for Pickering emulsions and Au nanoparticle/graphene oxide@polystyrene microspheres. Carbon N. Y. 2014, 71, 238–248. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Balme, S.; Bechelany, M.; Miele, P. Inverse Pickering Emulsion Stabilized by Exfoliated Hexagonal-Boron Nitride (h-BN). Langmuir 2017, 33, 13394–13400. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Pickering emulsions stabilized with two-dimensional (2D) materials: A comparative study. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 563, 183–192. [Google Scholar] [CrossRef]
- Yao, Y.; Lin, Z.; Li, Z.; Song, X.; Moon, K.S.; Wong, C.P. Large-scale production of two-dimensional nanosheets. J. Mater. Chem. 2012, 22, 13494–13499. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, X.; Chen, J.; Yang, F. Effect of graphene oxide concentration on the morphologies and antifouling properties of PVDF ultrafiltration membranes. J. Environ. Chem. Eng. 2013, 1, 349–354. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Gassara, S.; Cambedouzou, J.; Bechelany, M.; Miele, P. Development of novel h-BNNS/PVA porous membranes via Pickering emulsion templating. Green Chem. 2018, 20, 4319–4329. [Google Scholar] [CrossRef]
- Llanos, J.; Camarillo, R.; Pérez, Á.; Cañizares, P. Polymer supported ultrafiltration as a technique for selective heavy metal separation and complex formation constants prediction. Sep. Purif. Technol. 2010, 73, 126–134. [Google Scholar] [CrossRef]
- Zeng, J.X.; Ye, H.Q.; Huang, N.D.; Liu, J.F.; Zheng, L.F. Selective separation of Hg(II) and Cd(II) from aqueous solutions by complexation–ultrafiltration process. Chemosphere 2009, 76, 706–710. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Poerio, T. Comparison of polyethylenimine, polyacrylic acid and poly(dimethylamine-co-epichlorohydrin-co-ethylenediamme) in Cu2+ removal from wastewaters by polymer-assisted ultrafiltration. Desalination 2004, 162, 217–228. [Google Scholar] [CrossRef]
- Zamariotto, D.; Lakard, B.; Fievet, P.; Fatin-Rouge, N. Retention of Cu(II)– and Ni(II)–polyaminocarboxylate complexes by ultrafiltration assisted with polyamines. Desalination 2010, 258, 87–92. [Google Scholar] [CrossRef]
- Molinari, R.; Poerio, T.; Argurio, P. Selective separation of copper(II) and nickel(II) from aqueous media using the complexation–ultrafiltration process. Chemosphere 2008, 70, 341–348. [Google Scholar] [CrossRef]
- Kadioglu, S.I.; Yilmaz, L.; Aydogan, N.; Onder Ozbelge, H. Removal of Heavy Metals from Multicomponent Metal Mixtures by Polymer Enhanced Ultrafiltration: Effects of pH, Ionic Strength and Conformational Changes in Polymer Structure. Sep. Sci. Technol. 2010, 45, 1363–1373. [Google Scholar] [CrossRef]
- Li, C.-W.; Cheng, C.-H.; Choo, K.-H.; Yen, W.-S. Polyelectrolyte enhanced ultrafiltration (PEUF) for the removal of Cd(II): Effects of organic ligands and solution pH. Chemosphere 2008, 72, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, G.C.; Keizer, K.; Neomagus, H.W.J.P.; Krieg, H.M. Copper(II) removal from polluted water with alumina/chitosan composite membranes. J. Memb. Sci. 2002, 197, 147–156. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Trivunac, K.; Stevanovic, S. Effects of operating parameters on efficiency of cadmium and zinc removal by the complexation–filtration process. Desalination 2006, 198, 282–287. [Google Scholar] [CrossRef]
- Nafti, M.; Ennigrou, D.J.; Horchani-Naifar, K.; Ferid, M. Poly(Sodium-4-styrenesulfonate) assisted ultrafiltration for nickel and copper removal from aqueous solutions: Optimization several parameters. Desalin. Water Treat. 2019, 154, 39–48. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Exfoliation of Hexagonal Boron Nitride (h-BN) in Liquide Phase by Ion Intercalation. Nanomaterials 2018, 8, 716. [Google Scholar] [CrossRef] [Green Version]
- Gassara, S.; Chinpa, W.; Quemener, D.; Ben Amar, R.; Deratani, A. Pore size tailoring of poly(ether imide) membrane from UF to NF range by chemical post-treatment using aminated oligomers. J. Memb. Sci. 2013, 436, 36–46. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Application of interfacially polymerized polyamide composite membranes to isopropanol dehydration: Effect of membrane pre-treatment and temperature. J. Memb. Sci. 2014, 453, 384–393. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Memb. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural Characterization of Thin-Film Polyamide Reverse Osmosis Membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Shen, K.; Cheng, C.; Zhang, T.; Wang, X. High performance polyamide composite nanofiltration membranes via reverse interfacial polymerization with the synergistic interaction of gelatin interlayer and trimesoyl chloride. J. Memb. Sci. 2019, 588, 117192. [Google Scholar] [CrossRef]
- Nagarajan, S.; Abessolo Ondo, D.; Gassara, S.; Bechelany, M.; Balme, S.; Miele, P.; Kalkura, N.; Pochat-Bohatier, C. Porous Gelatin Membrane Obtained from Pickering Emulsions Stabilized by Graphene Oxide. Langmuir 2018, 34, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Mimoune, S.; Belazzougui, R.E.; Amrani, F. Purification of aqueous solutions of metal ions by ultrafiltration. Desalination 2007. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, X.; Huang, W.; Lawless, D.; Feng, X. Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep. Purif. Technol. 2016. [Google Scholar] [CrossRef]
- Palencia, M.; Rivas, B.L.; Pereira, E.; Hernández, A.; Prádanos, P. Study of polymer–metal ion–membrane interactions in liquid-phase polymer-based retention (LPR) by continuous diafiltration. J. Memb. Sci. 2009, 336, 128–139. [Google Scholar] [CrossRef]
- Konradi, R. Weak Polyacid Brushes: Synthesis, Swelling Behavior, Complex Formation and Micropatterning. Ph.D. Thesis, Fakultät für Angewandte Wissenschaften, University of Freiburg, Freiburg, Germany, 2005. [Google Scholar]
- Bowen, W.R.; Williams, P.M. Quantitative predictive modelling of ultrafiltration processes: Colloidal science approaches. Adv. Colloid Interface Sci. 2007, 134–135, 3–14. [Google Scholar] [CrossRef]
- De, S.; Bhattacharya, P.K. Modeling of ultrafiltration process for a two-component aqueous solution of low and high (gel-forming) molecular weight solutes. J. Memb. Sci. 1997, 136, 57–69. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Moreno-Villoslada, I.; Quiroz, E.; Muñoz, C.; Rivas, B.L. Use of ultrafiltration on the analysis of low molecular weight complexing molecules. Analysis of iminodiacetic acid at constant ionic strength. Anal. Chem. 2001, 73, 5468–5471. [Google Scholar] [CrossRef]
- Choe, T.B.; Masse, P.; Verdier, A.; Clifton, M.J. Membrane fouling in the ultrafiltration of polyelectrolyte solutions: Polyacrylic acid and bovine serum albumin. J. Memb. Sci. 1986, 26, 17–30. [Google Scholar] [CrossRef]
- Magnenet, C.; Jurin, F.E.; Lakard, S.; Buron, C.C.; Lakard, B. Polyelectrolyte modification of ultrafiltration membrane for removal of copper ions. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 435, 170–177. [Google Scholar] [CrossRef]
- Freitas, R.R.Q.; Gueorguiev, G.K.; De Brito Mota, F.; De Castilho, C.M.C.; Stafström, S.; Kakanakova-Georgieva, A. Reactivity of adducts relevant to the deposition of hexagonal BN from first-principles calculations. Chem. Phys. Lett. 2013, 583, 119–124. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nafti Mateur, M.; Gonzalez Ortiz, D.; Jellouli Ennigrou, D.; Horchani-Naifer, K.; Bechelany, M.; Miele, P.; Pochat-Bohatier, C. Porous Gelatin Membranes Obtained from Pickering Emulsions Stabilized with h-BNNS: Application for Polyelectrolyte-Enhanced Ultrafiltration. Membranes 2020, 10, 144. https://doi.org/10.3390/membranes10070144
Nafti Mateur M, Gonzalez Ortiz D, Jellouli Ennigrou D, Horchani-Naifer K, Bechelany M, Miele P, Pochat-Bohatier C. Porous Gelatin Membranes Obtained from Pickering Emulsions Stabilized with h-BNNS: Application for Polyelectrolyte-Enhanced Ultrafiltration. Membranes. 2020; 10(7):144. https://doi.org/10.3390/membranes10070144
Chicago/Turabian StyleNafti Mateur, Molka, Danae Gonzalez Ortiz, Dorra Jellouli Ennigrou, Karima Horchani-Naifer, Mikhael Bechelany, Philippe Miele, and Céline Pochat-Bohatier. 2020. "Porous Gelatin Membranes Obtained from Pickering Emulsions Stabilized with h-BNNS: Application for Polyelectrolyte-Enhanced Ultrafiltration" Membranes 10, no. 7: 144. https://doi.org/10.3390/membranes10070144