Study on the Concentration of Acrylic Acid and Acetic Acid by Reverse Osmosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
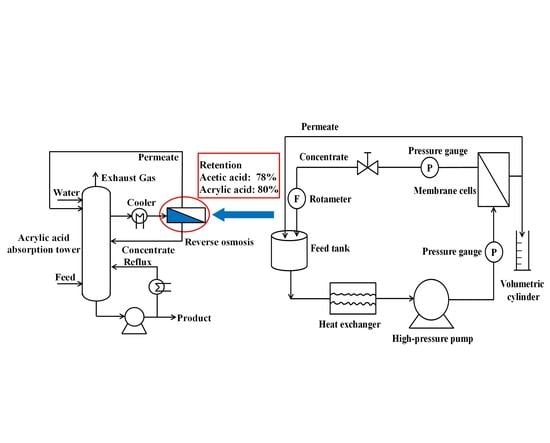
2.2. Filtration Experiment
2.3. Immersion Experiment
2.4. Analytical Methods
3. Results and Discussion
3.1. The Concentration Performance of the RO Membranes
3.1.1. Effect of Pressure
3.1.2. Effect of Temperature
3.1.3. Effect of Feed Concentration
3.2. Effect of Continuous Exposure to the Acid Solutions on the Performance and Characteristics of RO Membranes
3.2.1. Permeate Flux and Acid Retention
3.2.2. Membrane Surface Morphology
3.2.3. Membrane Surface Chemical Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Beerthuis, R.; Rothenberg, G.; Shiju, N.R. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables. Green Chem. 2015, 17, 1341–1361. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Yamada, Y.; Sato, S.; Ueda, W. Glycerol as a potential renewable raw material for acrylic acid production. Green Chem. 2017, 19, 3186–3213. [Google Scholar] [CrossRef]
- Grasselli, R.K.; Trifirò, F. Acrolein and acrylic acid from biomass. Rend. Lincei-Sci. Fis. 2017, 28, 59–67. [Google Scholar] [CrossRef]
- Straathof, A.J.; Sie, S.; Franco, T.T.; van der Wielen, L.A. Feasibility of acrylic acid production by fermentation. Appl. Microbiol. Biotechnol. 2005, 67, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, H. Highly selective production of acrylic acid from glycerol via two steps using Au/CeO2 catalysts. ACS Sustain. Chem. Eng. 2017, 5, 11371–11376. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y. Processes of producing acrylic acid by two-step process of propylene. Chem. Eng. Des. Commun. 2018, 44, 122–214. [Google Scholar]
- Li, A.; Dong, N.; He, M.; Pan, T. Evaluation of performance in a combined UASB and aerobic contact oxidation process treating acrylic wastewater. Environ. Technol. 2015, 36, 807–814. [Google Scholar] [CrossRef]
- Briegel, K.F.; Stanley, D.M.; James, E.; Jerrick, J.J.; Lydon, M.J. Method for Production of Aqueous (Meth)acrylic Acid. U.S. Patent 2009299095 A1, 25 October 2011. [Google Scholar]
- Min, Y.J.; Baek, S.W.; Song, J.H.; Kim, J.Y. Method of Continuous Recovery of (Meth)acrylic Acid and Apparatus for the Method. U.S. Patent 20190071383 A1, 3 July 2019. [Google Scholar]
- Aghapour Aktij, S.; Zirehpour, A.; Mollahosseini, A.; Taherzadeh, M.J.; Tiraferri, A.; Rahimpour, A. Feasibility of membrane processes for the recovery and purification of bio-based volatile fatty acids: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 24–40. [Google Scholar] [CrossRef]
- Lyu, H.; Chen, K.; Yang, X.; Younas, R.; Zhu, X.; Luo, G.; Zhang, S.; Chen, J. Two-stage nanofiltration process for high-value chemical production from hydrolysates of lignocellulosic biomass through hydrothermal liquefaction. Sep. Purif. Technol. 2015, 147, 276–283. [Google Scholar] [CrossRef]
- Baruah, K.; Hazarika, S. Separation of acetic acid from dilute aqueous solution by nanofiltration membrane. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Lakra, R.; Saranya, R.; Lukka Thuyavan, Y.; Sugashini, S.; Begum, K.M.M.S.; Arthanareeswaran, G. Separation of acetic acid and reducing sugars from biomass derived hydrosylate using biopolymer blend polyethersulfone membrane. Sep. Purif. Technol. 2013, 118, 853–861. [Google Scholar] [CrossRef]
- Ricci, B.C.; Ferreira, C.D.; Marques, L.S.; Martins, S.S.; Amaral, M.C.S. Assessment of nanofiltration and reverse osmosis potentialities to recover metals, sulfuric acid, and recycled water from acid gold mining effluent. Water Sci. Technol. 2016, 74, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Menchik, P.; Moraru, C.I. Nonthermal concentration of liquid foods by a combination of reverse osmosis and forward osmosis. Acid whey: A case study. J. Food Eng. 2019, 253, 40–48. [Google Scholar] [CrossRef]
- Wang, F.; Hao, H.; Sun, R.; Li, S.; Han, R.; Papelis, C.; Zhang, Y. Bench-scale and pilot-scale evaluation of coagulation pre-treatment for wastewater reused by reverse osmosis in a petrochemical circulating cooling water system. Desalination 2014, 335, 64–69. [Google Scholar] [CrossRef]
- Belkacem, M.; Bensadok, K.; Refes, A.; Charvier, P.M.; Nezzal, G. Water produce for pharmaceutical industry: Role of reverse osmosis stage. Desalination 2008, 221, 298–302. [Google Scholar] [CrossRef]
- González, M.P.; Navarro, R.; Saucedo, I.; Avila, M.; Revilla, J.; Bouchard, C. Purification of phosphoric acid solutions by reverse osmosis and nanofiltration. Desalination 2002, 147, 315–320. [Google Scholar] [CrossRef]
- Ricci, B.C.; Ferreira, C.D.; Aguiar, A.O.; Amaral, M.C.S. Integration of nanofiltration and reverse osmosis for metal separation and sulfuric acid recovery from gold mining effluent. Sep. Purif. Technol. 2015, 154, 11–21. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, C.; Wei, J. Simultaneous acetic acid separation and monosaccharide concentration by reverse osmosis. Bioresour. Technol. 2013, 131, 349–356. [Google Scholar] [CrossRef]
- Chen, C.; Ma, K.; Zhu, Q.; Tan, F.; Wang, Y.; He, M.; Hu, G. A method for concentration of monosaccharide and removal of inhibitors during hydrolysate pretreatment for improved bioethanol production. J. Clean. Prod. 2020, 260, 120999. [Google Scholar] [CrossRef]
- Ahsan, L.; Jahan, M.S.; Ni, Y. Recovering/concentrating of hemicellulosic sugars and acetic acid by nanofiltration and reverse osmosis from prehydrolysis liquor of kraft based hardwood dissolving pulp process. Bioresour. Technol. 2014, 155, 111–115. [Google Scholar] [CrossRef]
- Huifen, T.; Zhonghai, C.; Tao, L.; Ming, W. Study on the acid and alkali resistance of commercial seawater desalination RO membranes. Technol. Water Treat. 2016, 42, 70–74. [Google Scholar]
- Wang, M.; Wang, J.; Choong, Y. Simultaneous quantification of methanol and ethanol in alcoholic beverage using a rapid gas chromatographic method coupling with dual internal standards. Food Chem. 2004, 86, 609–615. [Google Scholar] [CrossRef]
- Esposito, G.G.; Schaefer, K.K. Gas chromatographic determination of acetic acid in industrial atmosphere and waste water. Am. Ind. Hyg. Assoc. J. 1976, 37, 268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wang, C.; Wei, J. Separation of acetic acid from monosaccharides by NF and RO membranes: Performance comparison. J. Membr. Sci. 2013, 429, 243–251. [Google Scholar] [CrossRef]
- Teella, A.; Huber, G.W.; Ford, D.M. Separation of acetic acid from the aqueous fraction of fast pyrolysis bio-oils using nanofiltration and reverse osmosis membranes. J. Membr. Sci. 2011, 378, 495–502. [Google Scholar] [CrossRef]
- Abejón, R.; Garea, A.; Irabien, A. Ultrapurification of hydrogen peroxide solution from ionic metals impurities to semiconductor grade by reverse osmosis. Sep. Purif. Technol. 2010, 76, 44–51. [Google Scholar] [CrossRef]
- Lyu, H.; Fang, Y.; Ren, S.; Chen, K.; Luo, G.; Zhang, S.; Chen, J. Monophenols separation from monosaccharides and acids by two-stage nanofiltration and reverse osmosis in hydrothermal liquefaction hydrolysates. J. Membr. Sci. 2016, 504, 141–152. [Google Scholar] [CrossRef]
- Nguyen, N.; Fargues, C.; Guiga, W.; Lameloise, M.L. Assessing nanofiltration and reverse osmosis for the detoxification of lignocellulosic hydrolysates. J. Membr. Sci. 2015, 487, 40–50. [Google Scholar] [CrossRef]
- Lvarez, E.A.; Zquez, G.V.; Nchez-Vilas, M.S.; Sanjurjo, B.A.; Navaza, J.M. Surface Tension of Organic Acids + Water Binary Mixtures from 20 °C to 50 °C. J. Chem. Eng. Data 1997, 42, 957–960. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Binder, K.; Kuhn, U.; Pöschl, U.; Su, H.; Cheng, Y. Molecular dynamics simulation of the surface tension of aqueous sodium chloride: From dilute to highly supersaturated solutions and molten salt. Atmos. Chem. Phys. 2018, 18, 17077–17086. [Google Scholar] [CrossRef] [Green Version]
- Duvel, W.A., Jr.; Helfgott, T. Removal of Wastewater Organics by Reverse Osmosis. J. Water Pollut. Control Fed. 1975, 47, 57–65. [Google Scholar] [PubMed]
- Takeuchi, K.; Takizawa, Y.; Kitazawa, H.; Fujii, M.; Hosaka, K.; Ortiz-Medina, J.; Morelos-Gomez, A.; Cruz-Silva, R.; Fujishige, M.; Akuzawa, N.; et al. Salt rejection behavior of carbon nanotube-polyamide nanocomposite reverse osmosis membranes in several salt solutions. Desalination 2018, 443, 165–171. [Google Scholar] [CrossRef]
- Agenson, K. Retention of a wide variety of organic pollutants by different nanofiltration/reverse osmosis membranes: Controlling parameters of process. J. Membr. Sci. 2003, 225, 91–103. [Google Scholar] [CrossRef]
- Breitner, L.N.; Howe, K.J.; Minakata, D. Effect of functional chemistry on the rejection of low-molecular weight neutral organics through reverse osmosis membranes for potable reuse. Environ. Sci. Technol. 2019, 53, 11401–11409. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E. The role of membrane surface charge and solute physico-chemical properties in the rejection of organic acids by NF membranes. J. Membr. Sci. 2005, 249, 227–234. [Google Scholar] [CrossRef]
- Sharma, R.R.; Agrawal, R.; Chellam, S. Temperature effects on sieving characteristics of thin-film composite nanofiltration membranes: Pore size distributions and transport parameters. J. Membr. Sci. 2003, 223, 69–87. [Google Scholar] [CrossRef]
- Nilsson, M.; Trägårdh, G.; Östergren, K. The influence of pH, salt and temperature on nanofiltration performance. J. Membr. Sci. 2008, 312, 97–106. [Google Scholar] [CrossRef]
- Ben Amar, N.; Saidani, H.; Deratani, A.; Palmeri, J. Effect of temperature on the transport of water and neutral solutes across nanofiltration membranes. Langmuir 2007, 23, 2937–2952. [Google Scholar] [CrossRef]
- Pino, L.; Vargas, C.; Schwarz, A.; Borquez, R. Influence of operating conditions on the removal of metals and sulfate from copper acid mine drainage by nanofiltration. Chem. Eng. J. 2018, 345, 114–125. [Google Scholar] [CrossRef]
- Platt, S.; Nyström, M.; Bottino, A.; Capannelli, G. Stability of NF membranes under extreme acidic conditions. J. Membr. Sci. 2004, 239, 91–103. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Gibert, O.; Torres, E.; Ayora, C.; Cortina, J.L. Application of nanofiltration for acidic waters containing rare earth elements: Influence of transition elements, acidity and membrane stability. Desalination 2018, 430, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Yun, T.; Chung, J.W.; Kwak, S. Recovery of sulfuric acid aqueous solution from copper-refining sulfuric acid wastewater using nanofiltration membrane process. J. Environ. Manag. 2018, 223, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xu, X.; Wu, J.; Li, C.; Chen, J.; Lv, B.; Zhu, B.; Xiang, H. SiO2-modified nanocomposite nanofiltration membranes with high flux and acid resistance. J. Appl. Polym. Sci. 2019, 136, 47436. [Google Scholar] [CrossRef]
- Hoseinpour, H.; Peyravi, M.; Nozad, A.; Jahanshahi, M. Static and dynamic assessments of polysulfonamide and poly(amide-sulfonamide) acid-stable membranes. J. Taiwan Inst. Chem. Eng. 2016, 67, 453–466. [Google Scholar] [CrossRef]
- Zhao, C.; Tang, C.Y.; Li, P.; Adrian, P.; Hu, G. Perfluorooctane sulfonate removal by nanofiltration membrane-the effect and interaction of magnesium ion/humic acid. J. Membr. Sci. 2016, 503, 31–41. [Google Scholar] [CrossRef]
- Do, V.T.; Tang, C.Y.; Reinhard, M.; Leckie, J.O. Degradation of polyamide nanofiltration and reverse osmosis membranes by hypochlorite. Environ. Sci. Technol. 2011, 46, 852–859. [Google Scholar] [CrossRef]







| Membranes | C5 | C6 |
|---|---|---|
| Active layer | polyamide (PA) | PA |
| Operating pH | 2–11 | 2–11 |
| Maximum operating temperature (°C) | 45 | 45 |
| Maximum operating pressure (MPa) | 8.27 | 8.27 |
| NaCl retention (%) | 99.8 a | 99.8 a |
| Permeate flow rate (m3/d) | 34.1 a | 45.4 a |
| Sample | Membrane | Solution Concentration |
|---|---|---|
| C5-Virgin a | C5 | - |
| C5-2.5 | C5 | 2.5% AA + 2.5% HAc |
| C5-7.5 | C5 | 7.5% AA + 7.5% HAc |
| C6-Virgin b | C6 | - |
| C6-2.5 | C6 | 2.5% AA + 2.5% HAc |
| C6-7.5 | C6 | 7.5% AA + 7.5% HAc |
| Sample | Average Roughness (nm) | ||
|---|---|---|---|
| 0 Month | 4 Months | 6 Months | |
| C5-2.5 | 137 | 127.5 | 117.0 |
| C5-7.5 | 137 | 108.6 | 103 |
| C6-2.5 | 87.2 | 86 | 78.9 |
| C6-7.5 | 87.2 | 83 | 76.2 |
| Sample | Time (Months) | Atomic Percent (at. %) | Atomic Ratio | |||
|---|---|---|---|---|---|---|
| N | C | O | C/O | O/N | ||
| C5-Virgin | 0 | 12.08 | 74.54 | 13.36 | 6.17 | 1.11 |
| C5-2.5 | 6 | 10.27 | 75.43 | 14.30 | 5.27 | 1.39 |
| C5-7.5 | 6 | 10.10 | 75.57 | 14.33 | 5.27 | 1.41 |
| C6-Virgin | 0 | 11.11 | 76.82 | 12.06 | 6.37 | 1.09 |
| C6-2.5 | 6 | 10.76 | 76.26 | 12.99 | 5.87 | 1.21 |
| C6-7.5 | 6 | 9.62 | 76.34 | 14.04 | 5.44 | 1.46 |
| Sample | Time (Months) | C 1s | N 1s | ||||
|---|---|---|---|---|---|---|---|
| 284.3 eV | 285.1 eV | 287.7 eV | 290.4 eV | 399.8 eV | 401.4 eV | ||
| C5-Virgin | 0 | 47.87 | 34.95 | 10.88 | 6.3 | 100 | - |
| C5-2.5 | 6 | 49.14 | 33.44 | 11.23 | 6.18 | 99.04 | 0.96 |
| C5-7.5 | 6 | 49.08 | 30.83 | 11.89 | 8.21 | 96.77 | 3.23 |
| C6-Virgin | 0 | 44.41 | 39.24 | 10.19 | 6.17 | 100 | - |
| C6-2.5 | 6 | 48.19 | 32.7 | 11.43 | 7.67 | 98.94 | 1.06 |
| C6-7.5 | 6 | 50.19 | 29.55 | 11.92 | 8.35 | 97.64 | 2.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Xie, L.; Du, H.; Xu, S.; Du, Y. Study on the Concentration of Acrylic Acid and Acetic Acid by Reverse Osmosis. Membranes 2020, 10, 142. https://doi.org/10.3390/membranes10070142
Liu Q, Xie L, Du H, Xu S, Du Y. Study on the Concentration of Acrylic Acid and Acetic Acid by Reverse Osmosis. Membranes. 2020; 10(7):142. https://doi.org/10.3390/membranes10070142
Chicago/Turabian StyleLiu, Qian, Lixin Xie, Hanxiao Du, Shichang Xu, and Yawei Du. 2020. "Study on the Concentration of Acrylic Acid and Acetic Acid by Reverse Osmosis" Membranes 10, no. 7: 142. https://doi.org/10.3390/membranes10070142