The Use of Succinonitrile as an Electrolyte Additive for Composite-Fiber Membranes in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Electrolyte Preparation
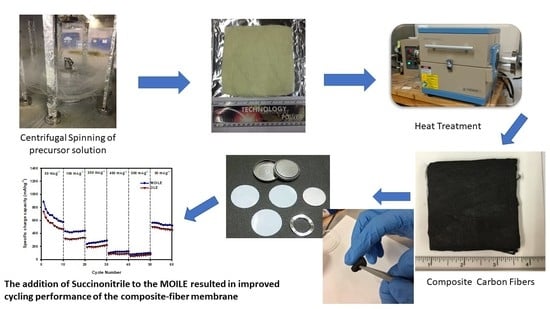
2.3. Preparation of SnO2/C Composite Fiber Membranes
2.4. Fiber Membrane Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Materials Characterization
3.2. Ionic Conductivity Measurement of Electrolytes at Different Temperature
3.3. Electrochemical Performance of A LiCoO2 Electrode in Lithium Cathode Half-Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Marcinek, M.; Syzdek, J.; Marczewski, M.; Piszcz, M.; Niedzicki, L.; Kalita, M.; Plewa-Marczewska, A.; Bitner, A.; Wieczorek, P.; Trzeciak, T.; et al. Electrolytes for Li-ion transport—Review. Solid State Ionics 2015, 276, 107–126. [Google Scholar] [CrossRef]
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer Electrolytes for Lithium-Ion Batteries: State of the Art and Perspectives. Chemsuschem 2015, 8, 2154–2175. [Google Scholar] [CrossRef]
- Quinzeni, I.; Ferrari, S.; Quartarone, E.; Tomasi, C.; Fagnoni, M.; Mustarelli, P. Li-doped mixtures of alkoxy-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)-imide and organic carbonates as safe liquid electrolytes for lithium batteries. J. Power Sources 2013, 237, 204–209. [Google Scholar] [CrossRef]
- Feng, X.N.; Zheng, S.Q.; Ren, D.S.; He, X.M.; Wang, L.; Liu, X.; Li, M.G.; Ouyang, M.G. Key Characteristics for Thermal Runaway of Li-ion Batteries. Energy Proced. 2019, 158, 4684–4689. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Kumar, T.P. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Feng, X.N.; Ouyang, M.G.; Liu, X.; Lu, L.G.; Xia, Y.; He, X.M. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Navarra, M.A. Ionic liquids as safe electrolyte components for Li-metal and Li-ion batteries. MRS Bull. 2013, 38, 548–553. [Google Scholar] [CrossRef]
- Lewandowski, A.; Swiderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries-An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Molinari, N.; Mailoa, J.P.; Kozinsky, B. General Trend of a Negative Li Effective Charge in Ionic Liquid Electrolytes. J. Phys. Chem. Lett. 2019, 10, 2313–2319. [Google Scholar] [CrossRef]
- Li, Z.; Smith, G.D.; Bedrov, D. Li+ Salvation and Transport Properties in Ionic Liquid/Lithium Salt Mixtures: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2012, 116, 12801–12809. [Google Scholar] [CrossRef]
- Bayley, P.M.; Lane, G.H.; Rocher, N.M.; Clare, B.R.; Best, A.S.; MacFarlane, D.R.; Forsyth, M. Transport properties of ionic liquid electrolytes with organic diluents. Phys. Chem. Chem. Phys. 2009, 11, 7202–7208. [Google Scholar] [CrossRef]
- Borodin, O.; Henderson, W.A.; Fox, E.T.; Berman, M.; Gobet, M.; Greenbaum, S. Influence of Solvent on Ion Aggregation and Transport in PY15TFSI Ionic Liquid-Aprotic Solvent Mixtures. J. Phys. Chem. B 2013, 117, 10581–10588. [Google Scholar] [CrossRef]
- Dong, D.P.; Bedrov, D. Charge Transport in [Li(tetraglyme)][bis(trifluoromethane) sulfonimide] Solvate Ionic Liquids: Insight from Molecular Dynamics Simulations. J. Phys. Chem. B 2018, 122, 9994–10004. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH: Hoboken, NJ, USA, 25 June 2008; Volume 1. [Google Scholar]
- Ji, L.W.; Lin, Z.; Alcoutlabi, M.; Zhang, X.W. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Villareal, J.; Alcoutlabi, M. Composite Nanofibers as Advanced Materials for Li-ion, Li-O-2 and Li-S Batteries. Electrochim. Acta 2016, 192, 529–550. [Google Scholar] [CrossRef] [Green Version]
- Villarreal, J.; Zuniga, L.; Valdez, A.; Alcoutlabi, M. The Use of Mixed Organic/Ionic Liquid Electrolytes with Forcespun Metal Oxides/Carbon Microfiber Electrodes in Lithium Ion Batteries. ECS Trans. 2018, 85, 387–394. [Google Scholar] [CrossRef]
- Garcia, B.; Lavallée, S.; Perron, G.; Michot, C.; Armand, M. Room temperature molten salts as lithium battery electrolyte. Electrochim. Acta 2004, 49, 4583–4588. [Google Scholar] [CrossRef]
- Guerfi, A.; Dontigny, M.; Charest, P.; Petitclerc, M.; Lagace, M.; Vijh, A.; Zaghib, K. Improved electrolytes for Li-ion batteries: Mixtures of ionic liquid and organic electrolyte with enhanced safety and electrochemical performance. J. Power Sources 2010, 195, 845–852. [Google Scholar] [CrossRef]
- Chopade, S.A.; Au, J.G.; Li, Z.; Schmidt, P.W.; Hillmyer, M.A.; Lodge, T.P. Robust Polymer Electrolyte Membranes with High Ambient-Temperature Lithium-Ion Conductivity via Polymerization-Induced Microphase Separation. ACS Appl. Mater. Interfaces 2017, 9, 14561–14565. [Google Scholar] [CrossRef]
- Alarco, P.J.; Abu-Lebdeh, Y.; Abouimrane, A.; Armand, M. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat. Mater. 2004, 3, 476–481. [Google Scholar] [CrossRef]
- Das, S.; Bhattacharyya, A.J. Influence of water and thermal history on ion transport in lithium salt-succinonitrile plastic crystalline electrolytes. Solid State Ionics 2010, 181, 1732–1739. [Google Scholar] [CrossRef]
- Wu, X.L.; Xin, S.; Seo, H.H.; Kim, J.; Guo, Y.G.; Lee, J.S. Enhanced Li+ conductivity in PEO-LiBOB polymer electrolytes by using succinonitrile as a plasticizer. Solid State Ionics 2011, 186, 1–6. [Google Scholar] [CrossRef]
- Fan, L.Z.; Hu, Y.S.; Bhattacharyya, A.J.; Maier, J. Succinonitrile as a versatile additive for polymer electrolytes. Adv. Funct. Mater. 2007, 17, 2800–2807. [Google Scholar] [CrossRef]
- Zha, W.P.; Chen, F.; Yang, D.J.; Shen, Q.; Zhang, L.M. High-performance Li6.4La3Zr1.4Ta0.6O12/Poly(ethylene oxide)/Succinonitrile composite electrolyte for solid-state lithium batteries. J. Power Sources 2018, 397, 87–94. [Google Scholar] [CrossRef]
- Chen, R.J.; Liu, F.; Chen, Y.; Ye, Y.S.; Huang, Y.X.; Wu, F.; Li, L. An investigation of functionalized electrolyte using succinonitrile additive for high voltage lithium-ion batteries. J. Power Sources 2016, 306, 70–77. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, T.H.; Lee, H.; Song, H.K. Electronegativity-induced enhancement of thermal stability by succinonitrile as an additive for Li ion batteries. Energy Environ. Sci. 2011, 4, 4038–4045. [Google Scholar] [CrossRef]
- Liu, C.F.; Neale, Z.G.; Cao, G.Z. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109–123. [Google Scholar] [CrossRef]
- Agubra, V.A.; De la Garza, D.; Gallegos, L.; Alcoutlabi, M. ForceSpinning of polyacrylonitrile for mass production of lithium-ion battery separators. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; De la Garza, D.; Gallegos, L.; Pokhrel, M.; Alcoutlabi, M. Forcespinning: A new method for the mass production of Sn/C composite nanofiber anodes for lithium ion batteries. Solid State Ionics 2016, 286, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Park, S.H.; Cho, J.K.; Lee, D.Y.; Park, T.J.; Lee, W.J.; Yang, K.S. Raman spectroscopic evaluation of polyacrylonitrile-based carbon nanofibers prepared by electrospinning. J. Raman Spectrosc. 2004, 35, 928–933. [Google Scholar] [CrossRef]
- Babu, V.S.; Seehra, M.S. Modeling of disorder and X-ray diffraction in coal-based graphitic carbons. Carbon 1996, 34, 1259–1265. [Google Scholar] [CrossRef]
- Flores, D.; Villarreal, J.; Lopez, J.; Alcoutlabi, M. Production of carbon fibers through Forcespinning (R) for use as anode materials in sodium ion batteries. Mater. Sci. Eng. B 2018, 236, 70–75. [Google Scholar] [CrossRef]
- Dahbi, M.; Ghamouss, F.; Tran-Van, F.; Lemordant, D.; Anouti, M. Comparative study of EC/DMC LiTFSI and LiPF6 electrolytes for electrochemical storage. J. Power Sources 2011, 196, 9743–9750. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Qin, C.D.; Yan, P.F.; Sui, M.L. Origins of capacity and voltage fading of LiCoO2 upon high voltage cycling. J. Mater. Chem. A 2019, 7, 20824–20831. [Google Scholar] [CrossRef]
- Logan, E.R.; Tonita, E.M.; Gering, K.L.; Li, J.; Ma, X.W.; Beaulieu, L.Y.; Dahn, J.R. A Study of the Physical Properties of Li-Ion Battery Electrolytes Containing Esters. J. Electrochem. Soc. 2018, 165, A21–A30. [Google Scholar] [CrossRef]
- Markovsky, B.; Talyossef, Y.; Salitra, G.; Aurbach, D.; Kim, H.J.; Choi, S. Cycling and storage performance at elevated temperatures of LiNi0.5Mn1.5O4 positive electrodes for advanced 5 VLi-ion batteries. Electrochem. Commun. 2004, 6, 821–826. [Google Scholar] [CrossRef]
- Kuhnel, R.S.; Lubke, M.; Winter, M.; Passerini, S.; Balducci, A. Suppression of aluminum current collector corrosion in ionic liquid containing electrolytes. J. Power Sources 2012, 214, 178–184. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarreal, J.; Orrostieta Chavez, R.; Chopade, S.A.; Lodge, T.P.; Alcoutlabi, M. The Use of Succinonitrile as an Electrolyte Additive for Composite-Fiber Membranes in Lithium-Ion Batteries. Membranes 2020, 10, 45. https://doi.org/10.3390/membranes10030045
Villarreal J, Orrostieta Chavez R, Chopade SA, Lodge TP, Alcoutlabi M. The Use of Succinonitrile as an Electrolyte Additive for Composite-Fiber Membranes in Lithium-Ion Batteries. Membranes. 2020; 10(3):45. https://doi.org/10.3390/membranes10030045
Chicago/Turabian StyleVillarreal, Jahaziel, Roberto Orrostieta Chavez, Sujay A. Chopade, Timothy P. Lodge, and Mataz Alcoutlabi. 2020. "The Use of Succinonitrile as an Electrolyte Additive for Composite-Fiber Membranes in Lithium-Ion Batteries" Membranes 10, no. 3: 45. https://doi.org/10.3390/membranes10030045