1. Introduction
The membrane bio-reactor (MBR) is a state-of-the-art technology that combines the activated sludge process with membrane filtration for the treatment of municipal and industrial wastewaters. Membrane bioreactors offer a series of advantages, such as high quality effluent, removal of pathogens, and diminishing the utilization of chemicals for disinfection, allowing it to be regarded as an environmentally friendly technology for wastewater treatment. However, membrane fouling still remains the major drawback in MBR systems preventing their universal application over the last few decades [
1]. Fouling is attributed to a variety of components present in the (waste) water, which increase the membrane’s resistance either by their adsorption or deposition onto its surface, or even by complete pore-blocking. Fouling leads to permeate flux decline, which in turn decreases the time intervals necessary for membrane cleaning and replacement, resulting in both higher capital and operating costs. According to the reversibility of flux, fouling can be (i) reversible, when it is removed by the application of physical cleaning methods; (ii) irreversible, when it is removed by the application of chemical cleaning methods; or (iii) irrecoverable, when it cannot be removed by the application of a physical or chemical cleaning method owing to the long-term use of the membrane after a series of consecutive cleaning cycles.
According to the nature of foulants, that is, the substances that cause membrane fouling, fouling can be also divided into (i) bio-fouling, owing to the formation of a bio-film layer, caused mainly by the presence of microorganisms that are attached and growing on the membrane surface; (ii) organic fouling, which is caused by organic compounds, such as polysaccharides, proteins, humic substances, and other organic bio-polymers; and (iii) inorganic fouling, which refers to the deposition of inorganic materials, like salts, metal oxides, and so on [
2]. Among the various compounds that exist in the activated sludge, the extracellular polymeric substances (EPSs) are considered to be the most significant foulants during the operation of MBR systems. EPSs include a variety of organic macromolecules, usually polysaccharides and proteins, which are located outside the bacteria cells or inside the microbial aggregates. They are classified into bound EPS (bEPS) or soluble EPS (sEPS), which are also known as soluble microbial products (SMPs). In particular, the carbohydrate fraction of SMP (SMP
c) has been often cited as the most significant constituent causing fouling in MBRs, although the role of protein compounds in fouling has not been yet fully elucidated [
3].
Over the last few years, several methods/techniques have been employed in order to control and/or mitigate membrane fouling in MBRs. These methods can be distinguished into conventional and innovative methods. The conventional methods have been implemented since the earlier years of MBR technology and include the operation of these systems under low fluxes; the application of physical methods, such as aeration with coarse bubbles, backwashing, and relaxation; as well as the application of chemical methods by utilization of simple chemical reagents solutions such as of NaOCl, citric acid, or oxalic acid. The innovative methods are implemented more recently and include the application of electric field, ultrasound, ozone, and various membrane surface modifications. It must be stated that, in most cases, the innovative methods are not applied individually, but they are usually implemented in combination with a conventional method, aiming to result in more effective reduction of fouling [
4,
5]. Regarding fouling characterization and quantification, various direct or indirect methods have been implemented, which include either standardized tests, such as the Delft filtration, the drainage test, the time-to-filer (TTF) method, and so on, or the measurement of specific foulants concentration, such as colloidal total organic carbon (TOC), SMP, and so on [
4,
6].
One of the most promising strategies for fouling control is the use of appropriate additives in the MBR, which alter the biomass characteristics, resulting in the improvement of the filtration process and increase of the membrane lifetime. The most commonly applied additives usually include coagulation/flocculation agents, adsorbents, and bio-film carriers (BFCs) or bio-carriers [
7,
8,
9,
10]. Dosing of coagulants into MBR systems has been reported to mitigate fouling owing to the reduction of SMP in the supernatant liquor and to the formation of large flocs, which limit the blockage of membranes pores [
11]. The coagulation agents, which are used for fouling control in MBRs, usually fall into three main categories: inorganic monomers, inorganic polymers, and organic polymers. The simultaneous adsorption and bio-degradation, rather than a single biological process, reflect the major advantage of using adsorbents in MBRs [
12]. Powdered activated carbon (PAC) is one of the most widely applied adsorption agents. Its addition to MBRs mitigates fouling by improving the removal of low molecular weight organics and EPS and SMP, which are considered to be primarily responsible for membrane fouling. PAC can also decrease the compressibility of sludge flocs and increase the porosity of cake layer, resulting in the increase of membrane flux [
9,
13,
14]. Bio-film carriers (BFCs) or bio-carriers are plastic or sponge-made materials that have been also reported to mitigate fouling by direct physical scouring of membrane and by reducing the amount of SMPs [
4].
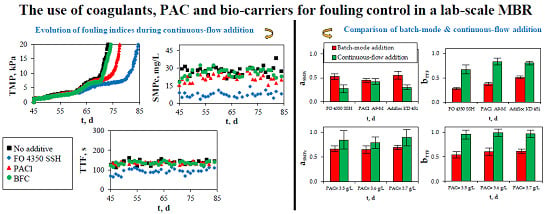
As shown above, coagulants/flocculants, PAC, and bio-carriers have been extensively used in MBRs for fouling control and/or mitigation. However, most studies concern batch-mode addition of these materials, making it difficult to assess the of fouling reduction in full-scale MBR applications where long-term operation is applied. In this study, the effect of coagulants, PAC, and bio-carriers on the membrane fouling of a lab-scale MBR is investigated and their performance during batch-mode and continuous-flow addition is compared. Furthermore, the present study is aimed at the development of a low-cost and time-saving methodology, through the evaluation of optimal additives based on the modification of the SMPc concentration and filterability tests. To the author’s best knowledge, this is the first study that systematically investigates and compares the anti-fouling potential of the most significant additive categories (at various dosages), including 14 commonly applied coagulation agents, PAC, and bio-film carriers.
2. Materials and Methods
2.1. Lab-Scale MBR Operation and Additives
The lab-scale MBR consisted of three main sub-units (
Figure 1a): (i) wastewater feed unit (200 L), (ii) bioreactor with submerged membrane (20 L), and (iii) permeate collection unit (40 L), and its operation was as follows (
Figure 1b): synthetic municipal wastewater (
Table 1), with a composition based on Organization for Economic Co-operation and Development (OECD) guidelines [
15], was fed as the substrate for the biological treatment process. The feed was led by a peristaltic pump to the aeration tank (bioreactor), where the concentration of the dissolved oxygen (DO) was monitored by a DO-meter in the range of 2–3 mg/L. The air needed for the biomass, as well as for the cleaning of the applied membrane, was supplied by an air compressor, the pressure of which was appropriately reduced to the desired value by means of an air pressure reducer/valve. Gas and liquid flow meters were applied for the measurement of aeration and feed or permeate flow rates, respectively, while the level of the mixed liquor in the membrane/aeration tank was controlled by level sensors. Another peristaltic pump was used to withdraw the permeate from the upper end of the membrane and a high-resolution pressure transmitter was employed in order to continuously record the evolution of trans-membrane pressure (TMP). The permeate collection unit was the final recipient of the produced permeate. A flat sheet, microfiltration membrane (Kubota Membranes Inc., Osaka, Japan) with a pore size of 0.4 μm and an effective area of 0.11 m
2 (made of chlorinated polyethylene) was used, while one-minute relaxation steps were performed every nine minutes of operation. This membrane type is commonly applied in MBR systems, as it is known to provide high rejection rates, owing to the gradual formation of a cake layer on the membrane surface that acts as a ‘secondary membrane’ and enhances the filtration process. It should also be stressed that the operation of the lab-scale MBR system was fully automated: a programmable logic controller (PLC) (Simatic S7-1200, SIEMENS, Nuremberg, Germany) was employed to control the operation of the peristaltic pumps, DO-meter, level sensors, and pressure transducer. Following the initial inoculation of the bioreactor with activated sludge, which was received from the municipal wastewater treatment plant of Thessaloniki (Northern Greece), the system was operated continuously in order to achieve steady-state conditions.
First, the batch-mode addition of 14 commercially available (commonly used) coagulation agents (
Table 2) and PAC (size > 100 μm, density: 0.4–0.5 g/cm
3, ash content ≤ 10%, humidity ≤ 4%) was conducted at different concentrations in appropriately received biomass samples. Bio-film carriers were not added to the samples, as the short-term nature of batch experiments would not allow the development of biomass onto their surface, which favors the adsorption of SMP
c. In order to examine the effect of solids retention time (SRT) on the process, the addition of coagulants and PAC was conducted at three SRTs (10, 15, and 20 days). During this phase (batch-mode addition experiments), which lasted for 384 days, activated sludge was not renewed and the acclimatization period for each SRT was determined as 2 × SRT, because this is generally considered to be an adequate amount of time for the adjustment of biomass to new conditions. The appropriate amount of sludge (2, 1.33, and 1 L) was daily withdrawn from the bioreactor (20 L), depending on the desired SRT (10, 15, and 20 days, respectively). The concentration of mixed liquor suspended solids (MLSS) in the bioreactor was 6.1 ± 1.1, 8.0 ± 1, and 9.2 ± 0.8 g/L, while organic loading, expressed by the ratio F/M (food to microorganisms), was approximately 0.20, 0.15, and 0.13 g chemical oxygen demand (COD)/g MLSS∙d, for SRT = 10, 15, and 20 days, respectively.
The additives that exhibited the best results during the batch-mode experiments were then continuously added to the bioreactor. Addition of optimal coagulants and PAC concentrations was conducted according to the following mass balance equation (Equation (1)), previously proposed by Gkotsis et al. [
16]:
where
is the additive mass flow rate into the bioreactor (kg/h),
is the additive mass that is accumulated in the aeration tank per time (kg/h), and
is the additive mass flow rate removed from the MBR (kg/h). During this phase (continuous-flow addition experiments), which lasted for 417 days, bio-film carriers were also added to the bioreactor at three filling ratios (40%, 50%, and 60%) and the activated sludge was renewed after the addition of different additives. In addition, the lab-scale MBR operated under constant SRT (20 days), after removing the appropriate amount of sludge (1 L) from the bioreactor (20 L) on a daily basis. The concentration of MLSS in the bioreactor was 10.8 ± 1.5 g/L, while F/M was approximately 0.11 g COD/g MLSS∙d.
It should also be stated that the membrane was chemically cleaned when the TMP reached the maximum TMP limit (20 kPa), however, a new membrane module was implemented when a new SRT was applied during the batch-mode addition or a new additive was tested during the continuous-flow addition.
2.2. Fouling Estimation
During both phases, irreversible fouling was assessed in terms of SMPc removal, according to the phenol-sulfuric acid method, and reversible fouling was assessed in terms of sludge filterability tests, according to the standard TTF (time-to-filter) method. Total fouling was estimated during the continuous-flow addition of the optimal additives by measuring the evolution of TMP.
SMPs were extracted by the following procedure: mixed liquor samples (50 mL) were obtained daily from the bioreactor and centrifuged (for 20 min at 2000 rpm) in order to separate the solid biomass. Then, for the determination of the carbohydrate fraction of SMPs in the supernatant, the phenol-sulfuric acid method was employed [
17], which is the most widely used colorimetric method for the determination of carbohydrate concentration in aqueous solutions. The principle of this method is that carbohydrates, when dehydrated by reaction with concentrated sulfuric acid, produce furfural derivatives. Further reaction between furfural derivatives and phenol develops a detectible color. A short description of the standard procedure is as follows: 1 mL aliquot of a carbohydrate solution was mixed with 1 mL of wt. 5% aqueous solution of phenol in a test tube. Subsequently, 5 mL of conc. H
2SO
4 was added rapidly to the mixture. After allowing the test tubes to stand for 10 min, they were vortexed for 30 s and placed for 20 min in a water bath at 25 °C for color development. Then, light absorption at 480 nm was recorded on a spectrophotometer. Reference solutions were prepared in identical manner as aforementioned, except that the 1 mL aliquot of carbohydrate was replaced by glucose. A Hitachi UV/vis double-beam spectrophotometer was used for these measurements. Irreversible fouling was assessed in terms of the following ratio (Equation (2)):
where a
SMPc is the ratio of SMP
c concentration after the addition of an additive (i.e., coagulant or PAC) in the mixed liquor, to the SMP
c concentration before this addition (i.e., the respective blank measurement). It is evident that the lower ratio a
SMPc indicates that the tested concentration is more effective in terms of SMP
c removal.
The time-to-filter (TTF) method is a well-established method that can be used as an easy and relatively rapid way to assess the mixed liquor filterability. During the TTF method, a Buchner funnel with a diameter of 90 mm and Whatman #1 and #2, that is, pore size of 11 μm and 8 μm, respectively, or equivalent filter papers are used (in the present study, Whatman #1 was used). Following its removal from the bioreactor, sludge amount of 200 mL is instantly poured on the Buchner funnel and the time required to obtain 100 mL of filtrate is measured at a vacuum pressure of 51 kPa (designated as TTF). It is understood that low TTF values indicate high biomass filterability, while high TTF values indicate low biomass filterability [
18,
19]. Reversible fouling was assessed in terms of the following ratio (Equation (3)):
Similarly, bTTF is the ratio of TTF recorded after the addition of an additive in the mixed liquor, to the TTF recorded before this addition, and the lower ratio bTTF indicates that sludge filterability is more enhanced. The sum of two ratios aSMPc + bTTF was also calculated in order to determine the additives that reduce both SMPc concentration and TTF the most and, consequently, are likely to mitigate both irreversible and reversible membrane fouling.
2.3. Determination of Effluent Quality Parameters
The quality parameters of the lab-scale MBR effluent (concentrations of COD, total nitrogen (TN), NH4+-N, and NO3−-N) were determined with standardized Hack–Lange LCK test kits (with part numbers 314, 238, 304, and 339, respectively), along with a DR-3900 spectrophotometer. Biochemical oxygen demand (BOD)5 was measured with a respirometric BOD5 system (Oxi700, Orbeco-Hellige, Sarasota, FL, USA). The present study is part of a scientific research project that focuses primarily on the influence of additives on membrane fouling and secondarily on the efficient removal of effluent quality parameters. Therefore, the lab-scale MBR system was not initially designed to include an anaerobic tank for promoting enhanced biological phosphorus removal, and thus phosphorus removal was not examined in the present work (however, a series of construction changes is currently being conducted in order to render the system more flexible, as well as to allow for the efficient phosphorus removal).
4. Conclusions
The present study examines the effect of different additives (coagulations agents, PAC, and bio-film carriers) on membrane fouling of a lab-scale MBR, as well as on COD, BOD5, and nitrogen removal and nitrification. Batch-mode experiments included the addition of coagulation agents and PAC to biomass samples, and SMPc and TTF modifications were employed as fouling indices. Continuous-flow experiments included the continuous, inline addition of the optimal coagulation agents and PAC to the bioreactor of the MBR and SMPc, TTF, and TMP modifications were employed as fouling indices. Bio-film carriers were also added during this phase. The results showed that the coagulation agents, which effectively reduced both the SMPc concentration and TTF during their batch-mode addition, were FO 4350 SSH at 10 mg/L, PAC1 A9-M at 100 mg Al/L, and Adifloc KD 451 at 10 mg/L. Similarly, the optimal PAC concentration was 3.6 ± 0.1 g/L. Continuous-flow addition of polyelectrolytes FO 4350 SSH and Adifloc KD 451 decreased SMPc concentration and TTF, resulting in the increase of membrane lifetime by 16% and 13%, respectively, as indicated by the evolution of TMP. On the contrary, fouling reduction with PAC addition was very low. It was interesting to notice that the addition of bio-film carriers at filling ratios of 40%, 50%, and 60% did not affect SMPc, TTF, and TMP. Finally, concerning the overall operation and effluent quality of MBR, COD, BOD5, and NH-N concentrations in the effluent were far below the respective legislation limits, rarely exceeding 30 mg/L, 15 mg/L, and 0.9 mg/L, respectively, and were not affected by the continuous-flow addition of coagulation agents and PAC.