Visible-Light Active Photocatalytic Dual Layer Hollow Fiber (DLHF) Membrane and Its Potential in Mitigating the Detrimental Effects of Bisphenol A in Water
Abstract
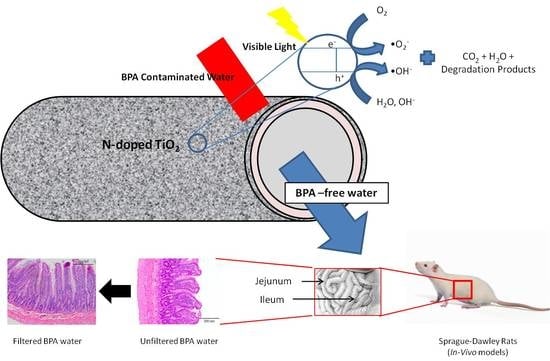
:1. Introduction
2. Methodology
2.1. Materials
2.1.1. Photocatalytic Dual Layer Hollow Fiber (DLHF) Membrane
2.1.2. In-Vivo Models
2.2. Fabrication of Photocatalytic Dual Layer Hollow Fiber Membrane
2.3. Membrane Properties Analysis
2.4. Photocatalytic Activity Evaluation
2.5. Animal Care and BPA Exposure
2.5.1. Dissection
2.5.2. Liver Function Test
2.5.3. Blood Pressure
2.5.4. Heamatoxylin and Eosin (H&E) Staining
3. Results and Discussion
3.1. Physical Properties of DLHF Membranes
3.2. Photocatalytic Degradation Evaluations
3.3. BPA-Treated Water Ameliorated Its Detrimental Effects in Comparison to BPA-Untreated Water
3.4. Blood Pressure (BP) Readings
3.5. Changes in Morphology of Jejunum and Ileum
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Grasselli, F.; Baratta, L.; Baioni, L.; Bussolati, S.; Ramoni, R.; Grolli, S.; Basini, G. Bisphenol A disrupts granulosa cell function. Domest. Anim. Endocrinol. 2010, 39, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Milić, N.; Četojević-Simin, D.; Milanović, M.; Sudji, J.; Milošević, N.; Ćurić, N.; Medić-Stojanoska, M. Estimation of in vivo and in vitro exposure to bisphenol A as food contaminant. Food Chem. Toxicol. 2015, 83, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Tsutsumi, O.; Ikezuki, Y.; Hiroi, H.; Osuga, Y.; Momoeda, M.; Taketani, Y. Estrogen Receptor-Mediated Effects of a Xenoestrogen, Bisphenol A, on Preimplantation Mouse Embryos. Biochem. Biophys. Res. Commun. 2000, 270, 918–921. [Google Scholar] [CrossRef]
- Le Corre, L.; Besnard, P.; Chagnon, M.-C. BPA, an Energy Balance Disruptor. Crit. Rev. Food Sci. Nutr. 2014, 55, 769–777. [Google Scholar] [CrossRef]
- Carwile, J.L.; Michels, K.B. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res. 2011, 111, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Brent, R.L. Bisphenol A and Obesity in Children and Adolescents. JAMA 2013, 309, 134. [Google Scholar] [CrossRef] [Green Version]
- Ranjit, N.; Siefert, K.; Padmanabhan, V. Bisphenol-A and disparities in birth outcomes: A review and directions for future research. J. Perinatol. 2009, 30, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Than, L.D.; Luong, N.S.; Ngo, V.D.; Tien, N.M.; Dung, T.N.; Nghia, N.M.; Lam, T.D. Highly Visible Light Activity of Nitrogen Doped TiO2 Prepared by Sol–Gel Approach. J. Electron. Mater. 2016, 46, 158–166. [Google Scholar] [CrossRef]
- Azami, M.S.; Nawawi, W.I.; Jawad, A.H.; Ishak, M.A.M.; Ismail, K. N-doped TiO2synthesised via microwave induced photocatalytic on RR4 dye removal under LED light irradiation. Sains Malays. 2017, 46, 1309–1316. [Google Scholar] [CrossRef]
- Lee, A.; Libera, J.A.; Waldman, R.Z.; Ahmed, A.; Avila, J.R.; Elam, J.W.; Darling, S.B. Conformal Nitrogen-Doped TiO2 Photocatalytic Coatings for Sunlight-Activated Membranes. Adv. Sustain. Syst. 2017, 1. [Google Scholar] [CrossRef] [Green Version]
- Kamaludin, R.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Visible Light-Driven Photocatalytic N-Doped Tio2 For Degradation of Bisphenol A (BPA) And Reactive Black 5 (RB5) Dye. Water Air Soil Pollut. 2018, 229, 363. [Google Scholar] [CrossRef]
- Molinari, R.; Palmisano, L.; Drioli, E.; Schiavello, M. Studies on various reactor configurations for coupling photocatalysis and membrane processes in water purification. J. Membr. Sci. 2002, 206, 399–415. [Google Scholar] [CrossRef]
- Braslavsky, S.A.; Braun, A.M.; Cassano, A.E.; Emeline, A.V.; Litter, M.; Palmisano, L.; Parmon, V.N.; Serpone, N. Glossary of terms used in photocatalysis and radiation catalysis (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 931–1014. [Google Scholar] [CrossRef] [Green Version]
- Ooi, T. Virtual Issue Posts on Organocatalysis: Design, Applications, and Diversity. ACS Catal. 2015, 5, 6980–6988. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Xia, C.; Jia, W.; Qing, W.; Zhang, W. Enhancing bioethanol productivity by a yeast-Immobilized catalytically active membrane in a fermentation-Pervaporation coupling process. J. Membr. Sci. 2020, 595, 117485. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Wang, Y. Nanoporous block copolymer membranes immobilized with gold nanoparticles for continuous flow catalysis. Polym. Chem. 2019. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Fabrication of Dual Layer Hollow Fibre Membranes for Photocatalytic Degradation of Organic Pollutants. Int. J. Chem. Eng. Appl. 2015, 6, 6–9. [Google Scholar] [CrossRef] [Green Version]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Morphological study of co-Extruded dual-Layer hollow fiber membranes incorporated with different TiO2 loadings. J. Membr. Sci. 2015, 479, 123–131. [Google Scholar] [CrossRef]
- Kamaludin, R.; Mohamad Puad, A.S.; Othman, M.H.D.; Kadir, S.H.S.A.; Harun, Z. Incorporation of N-doped TiO2 into dual layer hollow fiber (DLHF) membrane for visible light-Driven photocatalytic removal of reactive black 5. Polym. Test. 2019. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. Photocatalytic degradation of nonylphenol by immobilized TiO2 in dual layer hollow fibre membranes. Chem. Eng. J. 2015, 269, 255–261. [Google Scholar] [CrossRef]
- Kamaludin, R.; Othman, M.H.D.; Kadir, S.H.S.A.; Rahman, M.A.; Jaafar, J. The morphological properties study of photocatalytic TiO2/PVDF dual layer hollow fiber membrane for endocrine disrupting compounds degradation. Malays. J. Anal. Sci. 2017, 21. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Ayob NA, I.; Blanford, C.F.; Mohammad Rawi, N.F.; Szekely, G. Non-Woven Membrane Supports from Renewable Resources: Bamboo Fiber Reinforced Poly(Lactic Acid) Composites. ACS Sustain. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Yerzhankyzy, A.; Ghanem, B.S.; Wang, Y.; Alaslai, N.; Pinnau, I. Gas separation performance and mechanical properties of thermally-Rearranged polybenzoxazoles derived from an intrinsically microporous dihydroxyl-Functionalized triptycene diamine-Based polyimide. J. Membr. Sci. 2019, 117512. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Do, Y.S.; Lee, W.H.; Lee, M.J.; Guiver, M.D.; Lee, Y.M. High-Strength, soluble polyimide membranes incorporating Tröger’s Base for gas separation. J. Membr. Sci. 2016, 504, 55–65. [Google Scholar] [CrossRef]
- Ruzimuradov, O.; Nurmanov, S.; Hojamberdiev, M.; Prasad, R.M.; Gurlo, A.; Broetz, J.; Riedel, R. Fabrication of nitrogen-Doped TiO2 monolith with well-Defined macroporous and bicrystalline framework and its photocatalytic performance under visible light. J. Eur. Ceram. Soc. 2014, 34, 809–816. [Google Scholar] [CrossRef]
- Mago, G.; Kalyon, D.M.; Fisher, F.T. Membranes of Polyvinylidene Fluoride and PVDF Nanocomposites with Carbon Nanotubes via Immersion Precipitation. J. Nanomater. 2008, 2008, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, J.; Huo, Y. Highly Active TiO2N Photocatalysts Prepared by Treating TiO2Precursors in NH3/Ethanol Fluid under Supercritical Conditions. J. Phys. Chem. B 2006, 110, 1559–1565. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F. Structural characterization of N-Doped anatase–Rutile mixed phase TiO2 nanorods assembled microspheres synthesized by simple sol–Gel method. J. Sol-Gel Sci. Technol. 2015, 74, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO2 hybrid membranes with different dosage of nano-TiO2. J. Membr. Sci. 2012, 389, 522–531. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Nor, N.A.M. Photodegradation of phenol by N-Doped TiO2 anatase/rutile nanorods assembled microsphere under UV and visible light irradiation. Mater. Chem. Phys. 2015, 162, 113–123. [Google Scholar] [CrossRef]
- Kawamura, Y.; Etoh, M.; Hirakawa, Y.; Abe, Y.; Mutsuga, M. Bisphenol A in domestic and imported canned foods in Japan. Food Addit. Contam. Part A 2014, 31, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Zazouli, M.; Mahdavi, Y.; Bazrafshan, E.; Balarak, D. Phytodegradation potential of bisphenolA from aqueous solution by Azolla Filiculoides. J. Environ. Health Sci. Eng. 2014, 12, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- 2014 Updated Safety Assessment of Bisphenol A (BPA) for Use in Food Contact Applications 2014. Available online: https://www.fda.gov/media/90124/download (accessed on 22 January 2020).
- Desai, M.; Ferrini, M.G.; Han, G.; Jellyman, J.K.; Ross, M.G. In vivo maternal and in vitro BPA exposure effects on hypothalamic neurogenesis and appetite regulators. Environ. Res. 2018, 164, 45–52. [Google Scholar] [CrossRef]
- Szymanska, K.; Makowska, K.; Gonkowski, S. The influence of high and low doses of bisphenol a (BPA) on the enteric nervous system of the porcine ileum. Int. J. Mol. Sci. 2018, 19, 917. [Google Scholar] [CrossRef] [Green Version]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; vom Saal, F.S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef] [Green Version]
- Naji, H. The Interaction of C-Reactive Protein, Bisphenol A, & Cardiovascular Disease: A Demographical Analysis. Glob. J. Health Sci. 2017, 9, 63–72. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S. Relationship between serum cystatin C and hypertension among US adults without clinically recognized chronic kidney disease. J. Am. Soc. Hypertens. 2011, 5, 378–384. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.; Kim, J.H.; Lim, Y.-H.; Park, H.Y.; Hong, Y.-C. Associations of Bisphenol A Exposure With Heart Rate Variability and Blood Pressure. Hypertension 2012, 60, 786–793. [Google Scholar] [CrossRef]
- Patel, B.B.; Raad, M.; Sebag, I.A.; Chalifour, L.E. Sex-Specific cardiovascular responses to control or high fat diet feeding in C57bl/6 mice chronically exposed to bisphenol A. Toxicol. Rep. 2015, 2, 1310–1318. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Hong, Y.-C. Bisphenol A, Hypertension, and Cardiovascular Diseases: Epidemiological, Laboratory, and Clinical Trial Evidence. Curr. Hypertens. Rep. 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Apaydin, F.G.; Uzunhisarcikli, M.; Aslantürk, A.; Kalender, S. Bisphenol A-Induced Histopathological Alterations on Small Intestine Tissues of Rats: The Protective Role of Taurine and Curcumin. Environ. Sci. Pollutution Res. Int. 2018, 26, 12302–12310. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, A.; Ghorbel, H.; bouallegui, Z.; Marrekchi, R.; Isoda, H.; Sayadi, S. Oleuropein and hydroxytyrosol protect from bisphenol A effects in livers and kidneys of lactating mother rats and their pups’. Exp. Toxicol. Pathol. 2015, 67, 413–425. [Google Scholar] [CrossRef] [Green Version]









| DLHF Configuration | N-Doped TiO2/TiO2 P25 DLHF |
| Outer dope solution (wt%) | PVDF/N-doped TiO2 or TiO2 P25/DMAc (15/7.5/77.5) |
| Inner dope solution (wt%) | PVDF/PEG/DMAc (18/5/77) |
| Spinning Condition | |
| Outer dope flowrate (mL/min) | 2 |
| Inner dope flowrate (rpm) | 26 |
| Bore fluid | Distilled water |
| Bore fluid flow rate (mL/min) | 8 |
| Air gap (cm) | 10 |
| Take up speed (rpm) | 5 |
| Spinneret dimension (mm) | 0.8/1.2/2.6 |
| Outer dope flowrate (mL/min) | 2 |
| Configurations | N-Doped TiO2/PVDF DLHF | TiO2-P25/PVDF DLHF |
|---|---|---|
| Tensile Strength (MPa) | 13.3 ± 0.24 | 14.5 ± 1.54 |
| Elongation at Break (%) | 189.2 ± 4.43 | 223.02 ± 9.68 |
| Contact Angle (°) | 70.4 | 72.6 |
| Porosity | 35.1 | 37.9 |
| Water Flux (L/m2·h) | 59.90 | 67.19 |
| Band Gap (eV) | 2.64 | 2.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaludin, R.; Rasdi, Z.; Othman, M.H.D.; Abdul Kadir, S.H.S.; Mohd Nor, N.S.; Khan, J.; Wan Mohamad Zain, W.N.I.; Ismail, A.F.; A Rahman, M.; Jaafar, J. Visible-Light Active Photocatalytic Dual Layer Hollow Fiber (DLHF) Membrane and Its Potential in Mitigating the Detrimental Effects of Bisphenol A in Water. Membranes 2020, 10, 32. https://doi.org/10.3390/membranes10020032
Kamaludin R, Rasdi Z, Othman MHD, Abdul Kadir SHS, Mohd Nor NS, Khan J, Wan Mohamad Zain WNI, Ismail AF, A Rahman M, Jaafar J. Visible-Light Active Photocatalytic Dual Layer Hollow Fiber (DLHF) Membrane and Its Potential in Mitigating the Detrimental Effects of Bisphenol A in Water. Membranes. 2020; 10(2):32. https://doi.org/10.3390/membranes10020032
Chicago/Turabian StyleKamaludin, Roziana, Zatilfarihiah Rasdi, Mohd Hafiz Dzarfan Othman, Siti Hamimah Sheikh Abdul Kadir, Noor Shafina Mohd Nor, Jesmine Khan, Wan Nor I’zzah Wan Mohamad Zain, Ahmad Fauzi Ismail, Mukhlis A Rahman, and Juhana Jaafar. 2020. "Visible-Light Active Photocatalytic Dual Layer Hollow Fiber (DLHF) Membrane and Its Potential in Mitigating the Detrimental Effects of Bisphenol A in Water" Membranes 10, no. 2: 32. https://doi.org/10.3390/membranes10020032