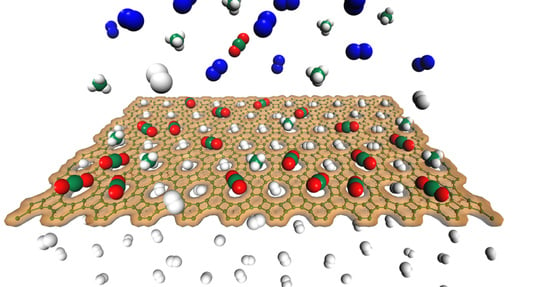
Enhanced Selective Hydrogen Permeation through Graphdiyne Membrane: A Theoretical Study
Abstract
:1. Introduction
2. Models and Methods
3. Results and Discussion
3.1. Gas Permeation Behavior
3.2. Transport Mechanism: Blocking Effect
3.3. Surface Charge Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Corredor, J.; Perez-Pena, E.; Rivero, M.J.; Ortiz, I. Performance of rGO/TiO2 photocatalytic membranes for hydrogen production. Membranes 2020, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Parvasi, P.; Mohammad Jokar, S.; Basile, A.; Iulianelli, A. An on-board pure H2 supply system based on a membrane reactor for a fuel cell vehicle: A theoretical study. Membranes 2020, 10, 159. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, P.; Liang, S.; Liu, B.; Shuai, Y.; Li, B. Exergy analysis of hydrogen production from steam gasification of biomass: A review. Int. J. Hydrog. Energy 2019, 44, 14290–14302. [Google Scholar] [CrossRef]
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V.; Barba, D. Hydrogen production via steam reforming: A critical analysis of MR and RMM technologies. Membranes 2020, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lee, W.H.; Seong, J.G.; Bae, J.Y.; Zhuang, Y.; Feng, S.; Wan, Y.; Lee, Y.M. Alicyclic segments upgrade hydrogen separation performance of intrinsically microporous polyimide membranes. J. Membr. Sci. 2020, 611, 118363. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Membranes for hydrogen separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef]
- Casadei, R.; Giacinti Baschetti, M.; Yoo, M.J.; Park, H.B.; Giorgini, L. Pebax@2533/graphene oxide nanocomposite membranes for carbon capture. Membranes 2020, 10, 188. [Google Scholar] [CrossRef]
- Castel, C.; Favre, E. Membrane separations and energy efficiency. J. Membr. Sci. 2018, 548, 345–357. [Google Scholar] [CrossRef]
- Richard, W. Baker. Overview of membrane science and technology. In Membrane Technology and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 1–14. [Google Scholar]
- Wang, S.; Yang, L.; He, G.; Shi, B.; Li, Y.; Wu, H.; Zhang, R.; Nunes, S.; Jiang, Z. Two-dimensional nanochannel membranes for molecular and ionic separations. Chem. Soc. Rev. 2020, 49, 1071–1089. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A new family of high-performance separation membranes. Angew. Chem. 2016, 55, 13384–13397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gupta, K.M.; Xu, Q.; Liu, G.; Jin, W. Gas permeation through double-layer graphene oxide membranes: The role of interlayer distance and pore offset. Sep. Purif. Technol. 2019, 209, 419–425. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Liu, G. Simulation of cations separation through charged porous graphene membrane. Chem. Phys. Lett. 2020, 753, 137606. [Google Scholar] [CrossRef]
- James, A.; John, C.; Owais, C.; Myakala, S.N.; Chandra Shekar, S.; Choudhuri, J.R.; Swathi, R.S. Graphynes: Indispensable nanoporous architectures in carbon flatland. RSC Adv. 2018, 8, 22998–23018. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Liu, H.; Wang, D.; Zhang, J. Graphdiyne: Synthesis, properties, and applications. Chem. Soc. Rev. 2019, 48, 908–936. [Google Scholar] [CrossRef]
- Rezaee, P.; Naeij, H.R. Graphenylene–1 membrane: An excellent candidate for hydrogen purification and helium separation. Carbon 2020, 157, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Xue, M.; Shen, C.; Zhang, Z.; Guo, W. Graphynes for water desalination and gas separation. Adv. Mater 2019, 31, e1803772. [Google Scholar] [CrossRef]
- Mahdizadeh, S.J.; Goharshadi, E.K. Multicomponent gas separation and purification using advanced 2D carbonaceous nanomaterials. RSC Adv. 2020, 10, 24255–24264. [Google Scholar] [CrossRef]
- Jiao, Y.; Du, A.; Hankel, M.; Zhu, Z.; Rudolph, V.; Smith, S.C. Graphdiyne: A versatile nanomaterial for electronics and hydrogen purification. Chem. Commun. 2011, 47, 11843–11845. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, X.; Zhang, Y.; Gao, H.; Wang, Y.; Shi, Q.; Rao, D.; Liu, Y.; Deng, K.; Lu, R. Graphdiyne as a high-efficiency membrane for separating oxygen from harmful gases: A first-principles study. ACS Appl. Mater. Interfaces 2016, 8, 28166–28170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sang, P.; Guo, S.; Liu, X.; Li, J.; Zhu, H.; Guo, W. Promising monolayer membranes for CO2/N2/CH4 separation: Graphdiynes modified respectively with hydrogen, fluorine, and oxygen atoms. Appl. Surf. Sci. 2017, 405, 455–464. [Google Scholar] [CrossRef]
- Apriliyanto, Y.B.; Lago, N.F.; Lombardi, A.; Evangelisti, S.; Bartolomei, M.; Leininger, T.; Pirani, F. Nanostructure selectivity for molecular adsorption and separation: The case of graphyne layers. J. Phys. Chem. C 2018, 122, 16195–16208. [Google Scholar] [CrossRef]
- Sang, P.; Zhao, L.; Xu, J.; Shi, Z.; Guo, S.; Yu, Y.; Zhu, H.; Yan, Z.; Guo, W. Excellent membranes for hydrogen purification: Dumbbell-shaped porous γ-graphynes. Int. J. Hydrog. Energy 2017, 42, 5168–5176. [Google Scholar] [CrossRef]
- Jiao, Y.; Du, A.; Smith, S.C.; Zhu, Z.; Qiao, S.Z. H2 purification by functionalized graphdiyne–role of nitrogen doping. J. Mater. Chem. A 2015, 3, 6767–6771. [Google Scholar] [CrossRef]
- Lei, G.; Liu, C.; Li, Q.; Xu, X. Graphyne nanostructure as a potential adsorbent for separation of H2S/CH4 mixture: Combining grand canonical Monte Carlo simulations with ideal adsorbed solution theory. Fuel 2016, 182, 210–219. [Google Scholar] [CrossRef]
- Bartolomei, M.; Giorgi, G. A nnovel nanoporous graphite based on graphynes: First-principles structure and carbon dioxide preferential physisorption. ACS Appl. Mater. Interfaces 2016, 8, 27996–28003. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; He, X.; Zhao, M.; Zhang, M.; Zhao, L.; Feng, X.; Luo, Y. Tunable hydrogen separation in sp-sp2 hybridized carbon membranes: A first-principles prediction. J. Phys. Chem. C 2012, 116, 16634–16638. [Google Scholar] [CrossRef]
- Bartolomei, M.; Carmona-Novillo, E.; Hernández, M.I.; Campos-Martínez, J.; Pirani, F.; Giorgi, G. Graphdiyne pores: “Ad Hoc” openings for helium separation applications. J. Phys. Chem. C 2014, 118, 29966–29972. [Google Scholar] [CrossRef] [Green Version]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, J.-H.; Ahn, J.-H. Graphene as a flexible electronic material: Mechanical limitations by defect formation and efforts to overcome. Mater. Today 2015, 18, 336–344. [Google Scholar] [CrossRef]
- Zhou, K.; Xu, Z. Renormalization of ionic solvation shells in nanochannels. ACS Appl. Mater. Interfaces 2018, 10, 27801–27809. [Google Scholar] [CrossRef]
- Wennberg, C.L.; Murtola, T.; Páll, S.; Abraham, M.J.; Hess, B.; Lindahl, E. Direct-space corrections enable fast and accurate Lorentz–Berthelot combination rule Lennard-Jones lattice summation. J. Chem. Theory Comput. 2015, 11, 5737–5746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [Green Version]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhou, S.; Sang, P.; Li, J.; Zhao, L. Inorganic graphenylene as a promising novel boron nitrogen membrane for hydrogen purification: A computational study. J. Mater. Sci. 2017, 52, 10285–10293. [Google Scholar] [CrossRef]
- Van Reis, R.; Zydney, A. Bioprocess membrane technology. J. Membr. Sci. 2007, 297, 16–50. [Google Scholar] [CrossRef]
- Omidvar, M.; Nguyen, H.; Liang, H.; Doherty, C.M.; Hill, A.J.; Stafford, C.M.; Feng, X.; Swihart, M.T.; Lin, H. Unexpectedly strong size-sieving ability in carbonized polybenzimidazole for membrane H2/CO2 separation. ACS Appl. Mater. Interfaces 2019, 11, 47365–47372. [Google Scholar] [CrossRef]
- Sun, Y.; Song, C.; Guo, X.; Liu, Y. Concurrent Manipulation of out-of-plane and regional in-plane orientations of NH2-UiO-66 membranes with significantly reduced anisotropic grain boundary and superior H2/CO2 separation performance. ACS Appl. Mater. Interfaces 2020, 12, 4494–4500. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Cheng, L.; Liu, G. Enhanced Selective Hydrogen Permeation through Graphdiyne Membrane: A Theoretical Study. Membranes 2020, 10, 286. https://doi.org/10.3390/membranes10100286
Liu Q, Cheng L, Liu G. Enhanced Selective Hydrogen Permeation through Graphdiyne Membrane: A Theoretical Study. Membranes. 2020; 10(10):286. https://doi.org/10.3390/membranes10100286
Chicago/Turabian StyleLiu, Quan, Long Cheng, and Gongping Liu. 2020. "Enhanced Selective Hydrogen Permeation through Graphdiyne Membrane: A Theoretical Study" Membranes 10, no. 10: 286. https://doi.org/10.3390/membranes10100286