Performance and Microbial Community of Different Biofilm Membrane Bioreactors Treating Antibiotic-Containing Synthetic Mariculture Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactors and Operating Conditions
2.2. Inoculated Sludge and Wastewater Composition
2.3. Analytical Methods
3. Results
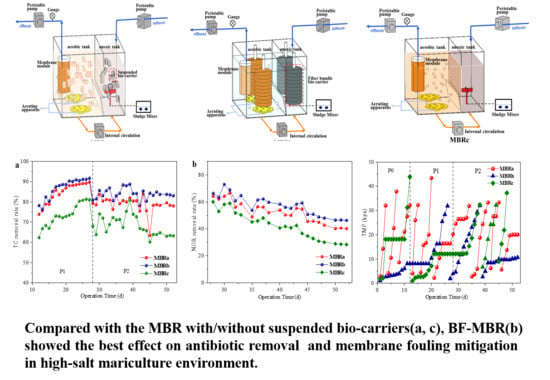
3.1. Reactor Performance
3.1.1. Removal of Bulk Pollutants
3.1.2. Removal of Antibiotics
3.2. Membrane Fouling Behavior during Continuous Operation
3.3. Microbial Community Dynamics
3.3.1. Microbial Diversity and Richness in the Three MBRs
3.3.2. Microbial Community Structure Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, D.; Chang, Q.; Li, Z.; Gao, M.; She, Z.; Wang, X.; Guo, L.; Zhao, Y.; Jin, C.; Gao, F. Performance and microbial community of a sequencing batch biofilm reactor treating synthetic mariculture wastewater under long-term exposure to norfloxacin. Bioresour. Technol. 2016, 222, 139–147. [Google Scholar] [CrossRef]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Lu, S.; Liu, X.; Liu, Y.; Xu, J.; Zhang, T.; Wang, Z.; Yang, Y. Occurrence of antibiotics and antibiotic resistance genes and their correlations in lower Yangtze River, China. Environ. Pollut. 2019. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ying, G.-G.; Singer, A.C.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, S.; Ye, C.; Lin, W.; Zhang, M.; Chen, L.; Li, J.; Yu, X. Biofilm processes in treating mariculture wastewater may be a reservoir of antibiotic resistance genes. Mar. Pollut. Bull. 2017, 118, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Thomas, Y.; Courties, C.; El Helwe, Y.; Herbland, A.; Lemonnier, H. Spatial and temporal extension of eutrophication associated with shrimp farm wastewater discharges in the New Caledonia lagoon. Mar. Pollut. Bull. 2010, 61, 387–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar] [CrossRef]
- Hagenbuch, I.M.; Pinckney, J.L. Toxic effect of the combined antibiotics ciprofloxacin, lincomycin, and tylosin on two species of marine diatoms. Water Res. 2012, 46, 5028–5036. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Otero, L.; Lema, J.M.; Omil, F. The effect and fate of antibiotics during the anaerobic digestion of pig manure. Bioresour. Technol. 2010, 101, 8581–8586. [Google Scholar] [CrossRef]
- Shimada, T.; Zilles, J.L.; Morgenroth, E.; Raskin, L. Inhibitory effects of the macrolide antimicrobial tylosin on anaerobic treatment. Biotechnol. Bioeng. 2008, 101, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.-G.; Chen, Q.-L.; Hu, H.-W.; He, J.-Z. Fate of antibiotic resistance genes during high-solid anaerobic co-digestion of pig manure with lignite. Bioresour. Technol. 2020, 303, 122906. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.; Ochoa, J.; Amrane, A. Biodegradation and biosorption of tetracycline and tylosin antibiotics in activated sludge system. Process. Biochem. 2009, 44, 1302–1306. [Google Scholar] [CrossRef]
- Gulkowska, A.; Leung, H.W.; So, M.K.; Taniyasu, S.; Yamashita, N.; Yeung, L.W.; Richardson, B.J.; Lei, A.P.; Giesy, J.P.; Lam, P.K. Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen, China. Water Res. 2008, 42, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, G.; Li, X.; Zou, S.; Li, P.; Hu, Z.; Li, J. Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res. 2007, 41, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Eichhorn, P.; Jensen, J.N.; Weber, A.S.; Aga, D.S. Removal of antibiotics in wastewater: Effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ. Sci. Technol. 2005, 39, 5816–5823. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Nghiem, L.D.; Liang, H. Can membrane bioreactor be a smart option for water treatment? Bioresour. Technol. Rep. 2018, 4, 80–87. [Google Scholar] [CrossRef]
- Shen, L.; Yuan, X.; Shen, W.; He, N.; Wang, Y.; Lu, H.; Lu, Y. Positive impact of biofilm on reducing the permeation of ampicillin through membrane for membrane bioreactor. Chemosphere 2014, 97, 34–39. [Google Scholar] [CrossRef]
- Song, W.; Li, Z.; Ding, Y.; Liu, F.; You, H.; Qi, P.; Wang, F.; Li, Y.; Jin, C. Performance of a novel hybrid membrane bioreactor for treating saline wastewater from mariculture: Assessment of pollutants removal and membrane filtration performance. Chem. Eng. J. 2018, 331, 695–703. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Jie, M.; Zhang, K.; Qian, Y.; Ma, J. Performance and microbial communities of different biofilm membrane bioreactors with pre-anoxic tanks treating mariculture wastewater. Bioresour. Technol. 2020, 295, 122302. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association Inc.: Washington, DC, USA, 1998. [Google Scholar]
- Ngoc Han, T.; Gin, K.Y.-H. Occurrence and removal of pharmaceuticals, hormones, personal care products, and endocrine disrupters in a full-scale water reclamation plant. Sci. Total. Environ. 2017, 599, 1503–1516. [Google Scholar] [CrossRef]
- Le, T.H.; Ng, C.; Tran, N.H.; Chen, H.; Gin, K.Y. Removal of antibiotic residues, antibiotic resistant bacteria and antibiotic resistance genes in municipal wastewater by membrane bioreactor systems. Water Res. 2018, 145, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, D.; Chu, W.; Li, M.; Lu, X. Nanoscaled magnetic CuFe2O4 as an activator of peroxymonosulfate for the degradation of antibiotics norfloxacin. Sep. Purif. Technol. 2019, 212, 536–544. [Google Scholar] [CrossRef]
- Schmidt, S.; Winter, J.; Gallert, C. Long-Term Effects of Antibiotics on the Elimination of Chemical Oxygen Demand, Nitrification, and Viable Bacteria in Laboratory-Scale Wastewater Treatment Plants. Arch. Environ. Contam. Toxicol. 2012, 63, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Sun, X.-F.; Xia, P.-F.; Wang, Y.-K.; Wang, S.-G. Investigation of fate and behavior of tetracycline in nitrifying sludge system. RSC Adv. 2015, 5, 87333–87340. [Google Scholar] [CrossRef]
- Ledala, N.; Wilkinson, B.J.; Jayaswal, R.K. Effects of oxacillin and tetracycline on autolysis, autolysin processing and atl transcription in Staphylococcus aureus. Int. J. Antimicrob. Agents 2006, 27, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xing, S.; Wang, X.; Wang, S. Changes of the reactor performance and the properties of granular sludge under tetracycline (TC) stress. Bioresour. Technol. 2013, 139, 170–175. [Google Scholar] [CrossRef]
- Meng, F.; Gao, G.; Yang, T.-T.; Chen, X.; Chao, Y.; Na, G.; Ge, L.; Huang, L.-N. Effects of fluoroquinolone antibiotics on reactor performance and microbial community structure of a membrane bioreactor. Chem. Eng. J. 2015, 280, 448–458. [Google Scholar] [CrossRef]
- Amorim, C.L.; Maia, A.S.; Mesquita, R.B.; Rangel, A.O.; van Loosdrecht, M.C.; Tiritan, M.E.; Castro, P.M. Performance of aerobic granular sludge in a sequencing batch bioreactor exposed to ofloxacin, norfloxacin and ciprofloxacin. Water Res. 2014, 50, 101–113. [Google Scholar] [CrossRef]
- Antón-Herrero, R.; García-Delgado, C.; Alonso-Izquierdo, M.; García-Rodríguez, G.; Cuevas, J.; Eymar, E. Comparative adsorption of tetracyclines on biochars and stevensite: Looking for the most effective adsorbent. Appl. Clay Sci. 2018, 160, 162–172. [Google Scholar] [CrossRef]
- Shi, Y.J.; Wang, X.H.; Qi, Z.; Diao, M.H.; Gao, M.M.; Xing, S.F.; Wang, S.G.; Zhao, X.C. Sorption and biodegradation of tetracycline by nitrifying granules and the toxicity of tetracycline on granules. J. Hazard. Mater. 2011, 191, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kummerer, K.; Al-Ahmad, A.; Mersch-Sundermann, V. Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere 2000, 40, 701–710. [Google Scholar] [CrossRef]
- Dorival-Garcia, N.; Zafra-Gomez, A.; Navalon, A.; Gonzalez, J.; Vilchez, J.L. Removal of quinolone antibiotics from wastewaters by sorption and biological degradation in laboratory-scale membrane bioreactors. Sci. Total Environ. 2013, 442, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, F.; Fu, Z.; Lei, R. Comparison between a moving bed membrane bioreactor and a conventional membrane bioreactor on membrane fouling. Bioresour. Technol. 2009, 100, 6655–6657. [Google Scholar] [CrossRef]
- Norton, J.M.; Klotz, M.G.; Stein, L.Y.; Arp, D.J.; Bottomley, P.J.; Chain, P.S.G.; Hauser, L.J.; Land, M.L.; Larimer, F.W.; Shin, M.W.; et al. Complete genome sequence of Nitrosospira multiformis, an ammonia-oxidizing bacterium from the soil environment. Appl. Environ. Microbiol. 2008, 74, 3559–3572. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zhang, Y.; Gao, Y.; Li, D.; Liu, R.; Liu, M.; Zhang, H.; Hu, B.; Yu, T.; Yang, M. Microbial Community Compositional Analysis for Series Reactors Treating High Level Antibiotic Wastewater. Environ. Sci. Technol. 2012, 46, 795–801. [Google Scholar] [CrossRef]
- Aydin, S.; Shahi, A.; Ozbayram, E.G.; Ince, B.; Ince, O. Use of PCR-DGGE based molecular methods to assessment of microbial diversity during anaerobic treatment of antibiotic combinations. Bioresour. Technol. 2015, 192, 735–740. [Google Scholar] [CrossRef]
- Stalder, T.; Alrhmoun, M.; Louvet, J.-N.; Casellas, M.; Maftah, C.; Carrion, C.; Pons, M.-N.; Pahl, O.; Ploy, M.-C.; Dagot, C. Dynamic Assessment of the Floc Morphology, Bacterial Diversity, and Integron Content of an Activated Sludge Reactor Processing Hospital Effluent. Envirion. Sci. Technol. 2013, 47, 7909–7917. [Google Scholar] [CrossRef]
- Westergaard, K.; Muller, A.K.; Christensen, S.; Bloem, J.; Sorensen, S.J. Effects of tylosin as a disturbance on the soil microbial community. Soil Biol. Biochem. 2001, 33, 2061–2071. [Google Scholar] [CrossRef]
- Yan, W.; Guo, Y.; Xiao, Y.; Wang, S.; Ding, R.; Jiang, J.; Gang, H.; Wang, H.; Yang, J.; Zhao, F. The changes of bacterial communities and antibiotic resistance genes in microbial fuel cells during long-term oxytetracycline processing. Water Res. 2018, 142, 105–114. [Google Scholar] [CrossRef]
- Kiely, P.D.; Rader, G.; Regan, J.M.; Logan, B.E. Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation endproducts. Bioresour. Technol. 2011, 102, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, A.; Margareto, A.; Robledo-Mahon, T.; Aranda, E.; Diaz-Cruz, S.; Gonzalez-Lopez, J.; Barcelo, D.; Vahala, R.; Gonzalez-Martinez, A. Performance and bacterial community structure of a granular autotrophic nitrogen removal bioreactor amended with high antibiotic concentrations. Chem. Eng. J. 2017, 325, 257–269. [Google Scholar] [CrossRef]
- Vyrides, I.; Andronikou, M.; Kyprianou, A.; Modic, A.; Filippeti, A.; Yiakoumis, C.; Samanides, C.G. CO2 conversion to CH4 using Zero Valent Iron (ZVI) and anaerobic granular sludge: Optimum batch conditions and microbial pathways. J. CO2 Util. 2018, 27, 415–422. [Google Scholar] [CrossRef]
- Rurangwa, E.; Verdegem, M.C.J. Microorganisms in recirculating aquaculture systems and their management. Rev. Aquac. 2015, 7, 117–130. [Google Scholar] [CrossRef]
- Chen, Y.; He, H.; Liu, H.; Li, H.; Zeng, G.; Xia, X.; Yang, C. Effect of salinity on removal performance and activated sludge characteristics in sequencing batch reactors. Bioresour. Technol. 2018, 249, 890–899. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, G.; Li, J.; Chen, S.-Y.; Li, Y.; Li, W.-T.; Li, A.-M. Response of performance and ammonia oxidizing bacteria community to high salinity stress in membrane bioreactor with elevated ammonia loading. Bioresour. Technol. 2016, 216, 714–721. [Google Scholar] [CrossRef]
- Pollet, T.; Berdjeb, L.; Garnier, C.; Durrieu, G.; Le Poupon, C.; Misson, B.; Jean-Francois, B. Prokaryotic community successions and interactions in marine biofilms: The key role of Flavobacteriia. Fems Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhang, T. Biodegradation and Adsorption of Antibiotics in the Activated Sludge Process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef]
- Martín-Moldes, Z.; Zamarro, M.T.; del Cerro, C.; Valencia, A.; Gómez, M.J.; Arcas, A.; Udaondo, Z.; García, J.L.; Nogales, J.; Carmona, M.; et al. Whole-genome analysis of Azoarcus sp. strain CIB provides genetic insights to its different lifestyles and predicts novel metabolic features. Syst. Appl. Microbiol. 2015, 38, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Li, Z.; Qian, J.; Shi, J.; Huang, L.; Tang, H.; Chen, X.; Lin, K.; Xu, P.; Liu, Y. Complete genome of Martelella sp AD-3, a moderately halophilic polycyclic aromatic hydrocarbons-degrading bacterium. J. Biotechnol. 2016, 225, 29–30. [Google Scholar] [CrossRef]





| Shannon Index | Chao1 Index | Good’s Coverage | |||||
|---|---|---|---|---|---|---|---|
| MBRa | MBRb | MBRc | MBRa | MBRb | MBRc | All Reactors | |
| P0 | 3.84 | 4.91 | 4.14 | 2741.59 | 3526.4 | 2971.25 | ≥0.98 |
| P1 | 3.00 | 3.62 | 3.16 | 1279.82 | 1590.43 | 1398.06 | 1.00 |
| P2 | 2.75 | 3.70 | 2.57 | 824.08 | 992.04 | 800.43 | ≥0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yuan, X.; Wang, H.; Ma, S.; Ji, B. Performance and Microbial Community of Different Biofilm Membrane Bioreactors Treating Antibiotic-Containing Synthetic Mariculture Wastewater. Membranes 2020, 10, 282. https://doi.org/10.3390/membranes10100282
Zhang H, Yuan X, Wang H, Ma S, Ji B. Performance and Microbial Community of Different Biofilm Membrane Bioreactors Treating Antibiotic-Containing Synthetic Mariculture Wastewater. Membranes. 2020; 10(10):282. https://doi.org/10.3390/membranes10100282
Chicago/Turabian StyleZhang, Huining, Xin Yuan, Hanqing Wang, Shuoqi Ma, and Bixiao Ji. 2020. "Performance and Microbial Community of Different Biofilm Membrane Bioreactors Treating Antibiotic-Containing Synthetic Mariculture Wastewater" Membranes 10, no. 10: 282. https://doi.org/10.3390/membranes10100282