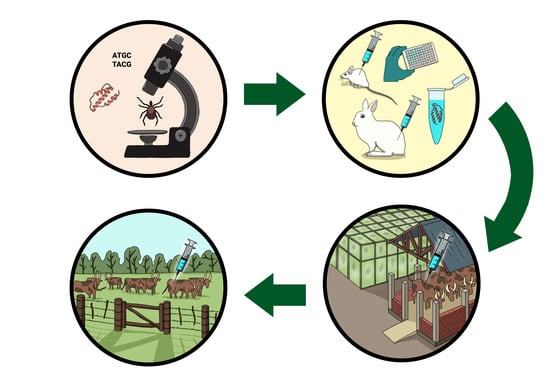
From Bench to Field: A Guide to Formulating and Evaluating Anti-Tick Vaccines Delving beyond Efficacy to Effectiveness
Abstract
:1. Introduction
What Is an Anti-Tick Vaccine?

2. The Basic Immunological Principle of Anti-Tick Vaccination
Hypothetical Mechanism of Anti-Tick Immune Memory Induction
3. Determination of Anti-Tick Vaccine Efficacy
4. Bottlenecks to Determining Anti-Tick Vaccine Efficacy
4.1. Determinants of Anti-Tick Vaccine Immune Response Induction
4.1.1. Adjuvant Anti-Tick Vaccine Formulation
4.1.2. Vaccine Dose Administration
- (A)
- Prime vaccine dose
- (B)
- Booster vaccine dose
4.1.3. Prime Boost Vaccination Strategy
- (A)
- Homologous prime boost dose vaccination
- (B)
- Heterologous prime boost dose vaccination
4.1.4. Vaccination Interval and Frequency
- (A)
- Vaccination booster frequency

- (B)
- Number of administered booster vaccine doses
4.1.5. Route of Vaccination
4.1.6. Vaccination Animal Model
5. Approach of Assessing Vaccine Efficacy
5.1. Assessing the Anti-Tick Vaccine Efficacy against Three-Host Ticks
- Step 1: Assess the humoral immune response kinetics
- Step 2: Assess adult tick engorgement
- Step 3: Vaccine effect on adult tick egg laying (oviposition)
- Determine the number of eggs by weight

- Step 4: Vaccine effect on egg hatchability
- (A)
- Independently pool and mix (e.g., using a paper) the eggs of ticks from each rabbit. Then, weigh a portion of eggs equivalent to the average cluster egg weight per tick (calculated in Step 2) and dispense the egg clusters into separate microtubes. Place the microtubes into separate bottles and incubate while slanted under appropriate conditions [137].
- (B)
- After the eggs have hatched, expose the molting larvae to white light [139] to ensure larvae migration from the egg shells to the sides of the bottle.
- (C)
- Randomly select one bottle of larvae from the corresponding rabbit within treatment groups to be used in subsequent infestation. Place the other bottles of larvae at −20 °C to freeze sterilize the larvae for at least 3 h. Remove the microtube with egg shells, pour the larvae on a Petri dish, weigh and count the larvae. Make a larvae count for at least three egg batches and calculate the average number of larvae. Then, weigh the other larvae. Based on the proportionality between the average larvae count and the weight of larvae from the remaining eggs, calculate the larvae count per egg batch. Calculate the standard deviation and the average larvae count per group. Given that an equal weight of eggs were incubated to obtain larvae, the average larvae count is representative of all egg clusters. Therefore, it is possible to extrapolate the number of larvae which is used for subsequent infestation.
- Step 5: Vaccine effect on larval feeding
- Step 6: Vaccine effect on larval molting
- Step 7: Vaccine effect on nymph feeding
- Step 8: Vaccine effect on adult tick engorgement and egg laying
- Step 9: Determine vaccine efficacy
- (A)
- Assess the vaccine effect on the feeding of female ticks
- (B)
- Assess the vaccine effect on egg laying
- (C)
- Assess the vaccine effect on egg hatchability
- (D)
- Assess vaccine effect on larvae feeding
- (E)
- Assess vaccine effect on nymph feeding
- (F)
- Calculate vaccine overall efficacy
5.2. Can the Approach Be Used to Assess Vaccine Efficacy against the One-Host Ticks?
- Step 1: Assess vaccine immunogenicity and prepare study ticks
- Step 2: Assess the vaccine effect on adult tick engorgement
- Step 3: Assess vaccine effect on adult tick egg laying (oviposition).
- Step 4: Assess vaccine effect on egg hatchability.
- Step 5: Assess vaccine effect on larvae to adult development.
- Step 6: Vaccine efficacy assessment
5.3. Foreseen Limitations and Opportunities of the Vaccine Efficacy Assessment Approach
6. Approach to Assessing Vaccine Effectiveness
6.1. Semi Field System Model for Conducting Anti-Tick Vaccine Effectiveness Studies
6.2. Assessment of Vaccine Effectiveness
- (A)
- Conduct experiments against all ixodid ticks (one-, two-, or three- host tick) using cattle.
- (B)
- In comparison to efficacy studies, conduct vaccine effectiveness studies with a greater number of ticks.
- (C)
- During each stage of tick infestation, collect and handle samples accordingly. This should also aid in monitoring the development of detached field ticks.
- (D)
- After each infestation stage, let the ticks to drop into the pasture and return the cattle to the shelter house.
- (E)
- Assess the vaccine effectiveness using two cattle populations. Figure 5 illustrates the vaccine effectiveness experimental design.
6.2.1. Determine Vaccine Effectiveness
- C1U: Ticks from unvaccinated cattle in population 1.
- C2U: Ticks from unvaccinated cattle in population 2.
- C1V: Ticks from vaccinated cattle in population 1.
- COVR: Overall tick from vaccinated and unvaccinated cattle population in 1.
6.2.2. Determining Vaccine Effectiveness against Three-Host Ticks
- What is the vaccine effectiveness on egg laying?
- What is the vaccine effectiveness on egg hatching?
- What is the vaccine effectiveness on larvae feeding?
- What is the vaccine effectiveness on nymph feeding?
6.2.3. Calculate the Vaccine Overall Effectiveness
- Determining vaccine effectiveness against one-host ticks
6.3. Foreseen Limitations and Opportunities of the Vaccine Effectiveness Assessment Approach
7. A Pipeline/Map for Development of Anti-Tick Vaccines
7.1. Phase I: Target Product Profiling
7.2. Phase II: Discovery/Feasibility Studies
7.3. Phase III: Early Phase Development
7.4. Licensure
7.5. Phase IV: Post Licensure/Late Phase Development
8. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Brites-Neto, J.; Duarte, K.M.; Martins, T.F. Tick-borne infections in human and animal population worldwide. Vet. World 2015, 8, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Samish, M.; Rehacek, J. Pathogens and predators of ticks and their potential in biological control. Annu. Rev. Entomol. 1999, 44, 159–182. [Google Scholar] [CrossRef]
- Horak, I.G.; Camicas, J.L.; Keirans, J.E. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida): A world list of valid tick names. Exp. Appl. Acarol. 2002, 28, 27–54. [Google Scholar] [CrossRef]
- Walker, A.R.; Bouattour, A.; Camicas, J.L.; Estrada-Pena, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bio-Science Reports: Edinburgh, UK, 2003. [Google Scholar]
- Guglielmone, A.A.; Robbins, R.G.; Apanaskevich, D.A.; Petney, T.N.; Estrada-Peña, A.; Horak, I.G.; Shao, R.; Barker, S.C. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: A list of valid species names. Zootaxa 2010, 25, 1–28. [Google Scholar] [CrossRef] [Green Version]
- McCoy, B.N.; Raffel, S.J.; Lopez, J.E.; Schwan, T.G. Bloodmeal size and spirochete acquisition of Ornithodoros hermsi (Acari: Argasidae) during feeding. J. Med. Entomol. 2010, 47, 1164–1172. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.R. Ticks and associated diseases: A retrospective review. Med. Vet. Entomol. 2014, 28, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Abbas, R.Z.; Zaman, M.A.; Colwell, D.D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 2014, 203, 6–20. [Google Scholar] [CrossRef]
- Graf, J.F.; Gogolewski, R.; Leach-Bing, N.; Sabatini, G.A.; Molento, M.B.; Bordin, E.L.; Arantes, G.J. Tick control: An industry point of view. Parasitology 2004, 129, S427–S442. [Google Scholar] [CrossRef] [PubMed]
- Manjunathachar, H.V.; Saravanan, B.C.; Kesavan, M.; Karthik, K.; Rathod, P.; Gopi, M.; Tamilmahanf, P.; Balarajud, B.L. Economic importance of ticks and their effective control strategies. Asian Pac. J. Trop. Dis. 2014, 4, 770–779. [Google Scholar] [CrossRef]
- De la Fuente, J.; Kocan, K.M. Strategies for development of vaccines for control of Ixodid tick species. Parasite Immunol. 2006, 28, 275–283. [Google Scholar] [CrossRef]
- Nuttall, P.A.; Trimnell, A.R.; Kazimirova, M.; Labuda, M. Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite Immunol. 2006, 28, 155–163. [Google Scholar] [CrossRef]
- Bensaci, M.; Bhattacharya, D.; Clark, R.; Hu, L.T. Oral vaccination with vaccinia virus expressing the tick antigen subolesin inhibits tick feeding and transmission of Borrelia burgdorferi. Vaccine 2012, 30, 6040–6046. [Google Scholar] [CrossRef] [Green Version]
- Labuda, M.; Trimnell, A.R.; Licková, M.; Kazimírová, M.; Davies, G.M.; Lissina, O.; Hails, R.S.; Nuttall, P.A. An antivector vaccine protects against a lethal vector-borne pathogen. PLoS Pathog. 2006, 2, e27. [Google Scholar] [CrossRef] [Green Version]
- Neelakanta, G.; Sultana, H. Transmission-blocking vaccines: Focus on anti-vector vaccines against tick-borne iseases. Arch. Immunol. Ther. Exp. 2015, 63, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Wikel, S.K.; Ramachandra, R.N.; Bergman, D.K. Tick-induced modulation of the host immune response. Int. J. Parasitol 1994, 24, 59–66. [Google Scholar] [CrossRef]
- Wikel, S.K. Host immunity to ticks. Annu. Rev. Entomol 1996, 41, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Brossard, M.; Wikel, S.K. Immunology of interactions between ticks and hosts. Med. Vet. Entomol. 1997, 11, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Brossard, M.; Wikel, S.K. Tick immunobiology. Parasitology 2004, 129, S161–S176. [Google Scholar] [CrossRef]
- Robbertse, L.; Richards, S.A.; Maritz-Olivier, C. Bovine immune factors underlying tick resistance: Integration and future directions. Front. Cell. Infect. Microbiol. 2017, 7, 522. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, C.; Narasimhan, S.; Vidyarthi, A.; Booth, C.J.; Mehta, S.; Meister, L.; Diktas, H.; Strank, N.; Lynn, G.E.; DePonte, K.; et al. Repeat tick exposure elicits distinct immune responses in guinea pigs and mice. Ticks Tick-Born Dis. 2020, 11, 101529. [Google Scholar] [CrossRef] [PubMed]
- Wambura, P.N.; Gwakisa, P.S.; Silayo, R.S.; Rugaimukamu, E.A. Breed-associated resistance to tick infestation in Bos indicus and their crosses with Bos taurus. Vet. Parasitol. 1998, 77, 63–70. [Google Scholar] [CrossRef]
- Rechav, Y. Naturally acquired resistance to ticks- A global view. Int. J. Trop. Insect. Sci. 1992, 13, 495–504. [Google Scholar] [CrossRef]
- Trager, W. Acquired immunity to ticks. J. Parasitol. 1939, 25, 57–81. [Google Scholar] [CrossRef]
- Anderson, J.M.; Moore, I.N.; Nagata, B.M.; Ribeiro, J.; Valenzuela, J.G.; Sonenshine, D.E. Ticks, Ixodes scapularis, feed repeatedly on white-footed mice despite strong inflammatory response: An expanding paradigm for understanding tick-host interactions. Front Immunol. 2017, 8, 1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, M.R.; Mèndez, L.; Valdez, M.; Redondo, M.; Espinosa, C.M.; Vargas, M.; Cruz, R.L.; Barrios, H.P.; Seoane, G.; Ramirez, E.S.; et al. Integrated control of Boophilus microplus ticks in Cuba based on vaccination with the anti-tick vaccine GavacTM. Exp. Appl. Acarol. 2004, 34, 375–382. [Google Scholar] [CrossRef]
- Andreotti, R. Performance of two Bm86 antigen vaccine formulation against tick using crossbreed bovines in stall test. Rev. Bras. Parasitol. Vet. 2006, 15, 97–100. [Google Scholar]
- García-García, J.C.; Gonzalez, I.L.; González, D.M.; Valdés, M.; Méndez, L.; Lamberti, J.; D’Agostino, B.; Citroni, D.; Fragoso, H.; Ortiz, M.; et al. Sequence variations in the Boophilus microplus Bm86 locus and implications for immunoprotection in cattle vaccinated with this antigen. Exp. Appl. Acarol. 1999, 23, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, H.; Rad, P.H.; Ortiz, M.; Rodríguez, M.; Redondo, M.; Herrera, L.; de la Fuente, J. Protection against Boophilus annulatus infestations in cattle vaccinated with the B. microplus Bm86-containing vaccine Gavac. Vaccine 1998, 16, 1990–1992. [Google Scholar] [CrossRef]
- De Vos, S.; Zeinstra, L.; Taoufik, A.; Willadsen, P.; Jongejan, F. Evidence for the utility of the Bm86 antigen from Boophilus microplus in vaccination against other tick species. Exp. Appl. Acarol. 2001, 25, 245–261. [Google Scholar] [CrossRef]
- De la Fuente, J.; Estrada-Peña, A. Why new vaccines for the control of ectoparasite vectors have not been registered and commercialized? Vaccines 2019, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Waldmann, H.; Munro, A.T. Cell-dependent mediator in the immune response. Nature 1973, 243, 356–357. [Google Scholar] [CrossRef]
- Lesinski, G.B.; Westerink, M.A. Novel vaccine strategies to T-independent antigens. J. Microbiol. Methods 2001, 47, 135–149. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A.; Araki, K.; Ahmed, R. From vaccines to memory and back. Immunity 2010, 33, 451–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetty, V.U.; Chaudhuri, P.; Sabella, C. Rationale for the immunization schedule: Why is it the way it is? Pediat. Rev. 2019, 40, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Abbas, A.K.; Lichtman, A.H.; Pilli, S. Cellular and Molecular Immunology, 9th ed.; Elsevier: Philadelphia, PA, USA, 2018; p. 565. ISBN 978-0-323-47978-3. [Google Scholar]
- Patarroyo, S.J.H.; de Sousa Neves, E.; Fidelis, C.F.; Tafur-Gómez, G.A.; de Araujo, L.; Vargas, M.I.; Sossai, S.; Prates-Patarroyo, P.A. Bovine immunisation with a recombinant peptide derived from synthetic SBm7462® (Bm86 epitope construct) immunogen for Rhipicephalus microplus control. Ticks Tick-Borne Dis. 2020, 11, 101461. [Google Scholar] [CrossRef]
- Ndawula, C., Jr.; Amaral Xavier, M.; Villavicencio, B.; Cortez Lopes, F.; Juliano, M.A.; Parizi, L.F.; Verli, H.; da Silva Vaz, I., Jr.; Ligabue-Braun, R. Prediction, mapping and validation of tick glutathione S-transferase B-cell epitopes. Ticks Tick-Borne Dis. 2020, 11, 101445. [Google Scholar] [CrossRef]
- Couto, J.; Seixas, G.; Stutzer, C.; Olivier, N.A.; Maritz-Olivier, C.; Antunes, S.; Domingos, A. Probing the Rhipicephalus bursa sialomes in potential anti-tick vaccine candidates: A reverse vaccinology approach. Biomedicines 2021, 9, 363. [Google Scholar] [CrossRef]
- Reichardt, P.; Dornbach, B.; Gunzer, M. APC, T cells, and the immune synapse. Curr. Top. Microbiol. Immunol. 2010, 340, 229–249. [Google Scholar] [CrossRef]
- Reeves, E.; James, E. Antigen processing and immune regulation in the response to tumours. Immunology 2017, 150, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Manz, R.A.; Thiel, A.; Radbruch, A. Lifetime of plasma cells in the bone marrow. Nature 1997, 388, 133–134. [Google Scholar] [CrossRef]
- Phan, T.G.; Tangye, S.G. Memory B cells: Total recall. Curr. Opin. Immunol. 2017, 45, 132–140. [Google Scholar] [CrossRef]
- Moran, I.; Nguyen, A.; Khoo, W.H.; Butt, D.; Bourne, K.; Young, C.; Hermes, J.R.; Biro, M.; Gracie, G.; Ma, C.S.; et al. Memory B cells are reactivated in subcapsular proliferative foci of lymph nodes. Nat. Commun. 2018, 9, 3372. [Google Scholar] [CrossRef] [PubMed]
- Jittapalapong, S.; Thanasilp, S.; Kaewmongkol, G.; Sirinarukmitr, T. The progress and process of the development of anti-tick vaccine against cattle ticks (Boophilus microplus) in Thailand. Kasetsart J. 2006, 40, 276–283. [Google Scholar]
- Shim, E.; Galvani, A.P. Distinguishing vaccine efficacy and effectiveness. Vaccine 2012, 30, 6700–6705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, M.; Yule, G.U. The statistics of anti-typhoid and anti-cholera inoculations, and the interpretation of such statistics in general. Proc. R Soc. Med. 1915, 8, 113–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikel, S.K. Immunomodulation of host responses to ectoparasite infestation-an overview. Vet. Parasitol. 1984, 14, 321–339. [Google Scholar] [CrossRef]
- Cunha, R.C.; Andreotti, R.; Garcia, M.V.; Aguirre, A.A.R.; Leitão, A. Calculation of the efficacy of vaccines against tick infestations on cattle. Rev. Bras. Parasitol. Vet. 2013, 22, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, A.; Garcia, M.V.; Szabó, M.P.; Barros, J.C.; Andreotti, R. Formula to evaluate efficacy of vaccines and systemic substances against three-host ticks. Int. J. Parasitol. 2015, 45, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Szabó, M.P.J.; Bechara, G.H. Immunisation of dogs and guinea pigs against Rhipicephalus sanguineus ticks using gut extract. Vet. Parasitol. 1997, 68, 283–294. [Google Scholar] [CrossRef]
- Kasaija, P.D.; Contreras, M.; Kabi, F.; Mugerwa, S.; de la Fuente, J. Vaccination with recombinant Subolesin antigens provides cross-tick species protection in Bos indicus and crossbred cattle in Uganda. Vaccines 2020, 8, 319. [Google Scholar] [CrossRef]
- Schetters, T.; Bishop, R.; Crampton, M.; Kopáček, P.; Lew-Tabor, A.; Maritz-Olivier, C.; Miller, R.; Mosqueda, J.; Patarroyo, J.; Rodriguez-Valle, M.; et al. Cattle tick vaccine researchers join forces in CATVAC. Parasite Vectors 2016, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Ndawula, C., Jr.; Tabor, A.E. Cocktail anti-tick vaccines: The unforeseen constraints and approaches toward enhanced efficacies. Vaccines 2020, 8, 457. [Google Scholar] [CrossRef]
- Simionatto, S.; Marchioro, S.B.; Galli, V.; Hartwig, D.D.; Carlessi, R.M.; Munari, F.M.; Laurino, J.P.; Conceição, F.R.; Dellagostin, O.A. Cloning and purification of recombinant proteins of Mycoplasma hyopneumoniae expressed in Escherichia coli. Protein Expr. Purif. 2010, 69, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D. Vaccine adjuvants: Why and how. Hum. Vaccin. Immunother. 2016, 12, 2709–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallovic, M.D.; Montjoy, D.G.; Collier, M.A.; Do, C.; Wyslouzil, B.E.; Bachelder, E.M.; Ainslie, K.M. Chemically modified inulin microparticles serving dual function as a protein antigen delivery vehicle and immunostimulatory adjuvant. Biomater. Sci. 2016, 4, 483–493. [Google Scholar] [CrossRef]
- Mohan, T.; Verma, P.; Rao, D.N. Novel adjuvants and delivery vehicles for vaccines development: A road ahead. Ind. J. Med. Res. 2013, 138, 779–795. [Google Scholar]
- Apostólico, J.; Lunardelli, V.A.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, modus operandi, and licensing. J. Immunol. Res. 2016, 2016, 1459394. [Google Scholar] [CrossRef] [Green Version]
- Herbert, W.J. The mode of action of mineral-oil emulsion adjuvants on antibody production in mice. Immunology 1968, 14, 301–318. [Google Scholar] [PubMed]
- Chauhan, N.; Tiwari, S.; Iype, T.; Jain, U. An overview of adjuvants utilized in prophylactic vaccine formulation as immunomodulators. Expert Rev. Vaccines 2017, 16, 491–502. [Google Scholar] [CrossRef]
- Brown, S.J.; Shapiro, S.Z.; Askenase, P.W. Characterization of tick antigens inducing host immune resistance. I. Immunization of guinea pigs with Amblyomma americanum-derived salivary gland extracts and identification of an important salivary gland protein antigen with guinea pig anti-tick antibodies. J. Immunol. 1984, 33, 3319–3325. [Google Scholar]
- Marois, I.; Cloutier, A.; Garneau, É.; Richter, M.V. Initial infectious dose dictates the innate, adaptive, and memory responses to influenza in the respiratory tract. J. Leukoc. Biol. 2012, 92, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.; Li, Y.; McKay, B.; Pawelek, K.A.; Zarnitsyna, V.; Antia, R. Exploring the impact of inoculum dose on host immunity and morbidity to inform model-based vaccine design. PLoS Comput. Biol. 2018, 14, e1006505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taborda, C.P.; Rivera, J.; Zaragoza, O.; Casadevall, A. More is not necessarily better: Prozone-like effects in passive immunization with IgG 1. J. Immunol. 2003, 170, 3621–3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billeskov, R.; Lindenstrom, T.; Woodworth, J.; Vilaplana, C.; Cardona, P.J.; Cassidy, J.P.; Mortensen, R.; Agger, E.M.; Andersen, P. High antigen dose is detrimental to post-exposure vaccine protection against tuberculosis. Front. Immunol. 2017, 8, 1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, B. Studies on the regulation of avidity at the level of the single antibody-forming cell the effect of antigen dose and time after immunization. J. Exp. Med. 1970, 132, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Siskind, G.W.; Dunn, P.; Walker, J.G. Studies on the control of antibody synthesis. II. Effect of antigen dose and of suppression by passive antibody on the affinity of antibody synthesized. J. Exp. Med. 1968, 127, 55–66. [Google Scholar] [CrossRef]
- Goidl, E.A.; Paul, W.E.; Siskind, G.W.; Benacerraf, B. The effect of antigen dose and time after immunization on the amount and affinity of anti-hapten antibody. J. Immunol. 1968, 100, 371–375. [Google Scholar]
- Eisen, H.N.; Siskind, G.W. Variations in affinities ofantibodies during the immune response. Biochemistry 1964, 3, 996–1008. [Google Scholar] [CrossRef]
- Theis, G.A.; Siskind, G.W. Selection of cell populations in induction of tolerance: Affinity of antibody formed in partially tolerant rabbits. J. Immunol. 1968, 100, 138–141. [Google Scholar]
- Paul, W.E.; Siskind, G.W.; Benacerraf, B. A study of the “termination” of tolerance to BSA with DNP-BSA in rabbits: Relative affinities of the antibodies for the immunizing and paralyzing antigens. Immunology 1967, 13, 147. [Google Scholar] [PubMed]
- Hu, Z.; Molloy, M.J.; Usherwood, E.J. CD4(+) T-cell dependence of primary CD8(+) T-cell response against Vaccinia virus depends upon route of infection and viral dose. Cell. Mol. Immunol. 2016, 13, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; O’Hagan, D.T. Recent advances in vaccine adjuvants. Pharm. Res. 2002, 19, 715–728. [Google Scholar] [CrossRef]
- Hum, B.A.L.; Chantler, S.M. Production of reagent antibodies. Methods Enzym. 1980, 70, 104–142. [Google Scholar] [CrossRef]
- Hanly, W.C.; Artwohl, J.E.; Bennett, B.T. Review of polyclonal antibody production procedures in mammals and poultry. ILAR J. 1995, 37, 93–118. [Google Scholar] [CrossRef] [Green Version]
- What’s the Right “Dose” of a Vaccine for Small-Breed Dogs? Available online: https://skeptvet.com/Blog/2016/01/whats-the-right-dose-of-a-vaccine-for-small-breed-dogs/ (accessed on 23 December 2020).
- Benet, L.Z.; Zia-Amirhosseini, P. Basic principles of pharmacokinetics. Toxicol. Pathol. 1995, 23, 115–123. [Google Scholar] [CrossRef]
- Jackson, L.A.; Opdebeeck, J.P. The effect of antigen concentration and vaccine regimen on the immunity induced by membrane antigens from the midgut of Boophilus microplus. Immunology 1989, 68, 272–276. [Google Scholar]
- Ndawula, C.J.; Sabadin, G.A.; Parizi, L.F.; da Silva Vaz, I.J. Constituting a glutathione S-transferase-cocktail vaccine against tick infestation. Vaccine 2019, 37, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Lam, K.P.; Rajewsky, K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature 2000, 407, 636–642. [Google Scholar] [CrossRef]
- Svehag, S.E.; Mandel, B. The formation and properties of poliovirus-neutralizing antibody. II. 19S and 7S antibody formation: Differences in antigen dose requirement for sustained synthesis, anamnesis, and sensitivity to x-irradiation. J. Exp. Med. 1964, 119, 21–39. [Google Scholar] [CrossRef] [Green Version]
- Hanna, M.G., Jr.; Peters., L.C. Requirement for continuous antigenic stimulation in the development and differentiation of antibody-forming cells: Effect of antigen dose. Immunology 1971, 20, 707–718. [Google Scholar]
- Trager, W. Further observations on acquired immunity to the tick Dermacentor variabilis Say. J. Parasitol. 1939, 25, 137–139. [Google Scholar] [CrossRef]
- Baxter, D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. 2007, 57, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Clark, T.G.; Cassidy-Hanley, D. Recombinant subunit vaccines: Potentials and constraints. Dev. Biol. 2005, 121, 153–163. [Google Scholar]
- Woodland, D.L. Jump-starting the immune system: Prime-boosting comes of age. Trends Immunol. 2004, 25, 98–104. [Google Scholar] [CrossRef]
- Nolz, J.C.; Harty, J.T. Strategies and implications for prime-boost vaccination to generate memory CD8 T cells. Adv. Exp. Med. Biol. 2011, 780, 69–83. [Google Scholar] [CrossRef]
- Ramshaw, I.A.; Ramsay, A.J. The prime-boost strategy: Exciting prospects for improved vaccination. Immunol. Today 2000, 21, 163–165. [Google Scholar] [CrossRef]
- Lu, S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009, 21, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Newman, M.J. Heterologous prime-boost vaccination strategies for HIV-1: Augmenting cellular immune responses. Curr. Opin. Investig. Drugs 2002, 3, 374–378. [Google Scholar] [PubMed]
- Kardani, K.; Bolhassani, A.; Shahbazihttp, S. Prime-boost vaccine strategy against viral infections: Mechanisms and benefits. Vaccine 2016, 34, 413–423. [Google Scholar] [CrossRef]
- Agrawal, B. Heterologous Immunity: Role in natural and vaccine-induced resistance to Infections. Front. Immunol. 2019, 10, 2631. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.; Gray, D. Immunological memory and protective immunity: Understanding their relation. Science 1996, 272, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.A.; Wang, Y.; Zhou, Y.; Cao, J.; Zhang, H.; Zhou, J. Cross protection induced by combined Subolesin-based DNA and protein immunizations against adult Haemaphysalis longicornis. Vaccine 2020, 38, 907–915. [Google Scholar] [CrossRef]
- Willadsen, P.; Riding, G.A.; McKenna, R.V.; Kemp, D.H.; Tellam, R.L.; Nielsen, J.N.; Lahnstein, J.; Cobon, G.S.; Gough, J.M. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J. Immunol. 1989, 143, 1346–1351. [Google Scholar] [PubMed]
- Hernandez, E.P.; Kusakisako, K.; Talactac, M.R.; Galay, R.L.; Hatta, T.; Matsuo, T.; Fujisaki, K.; Tsuji, N.; Tanaka, T. Characterization and expression analysis of a newly identified glutathione S-transferase of the hard tick Haemaphysalis longicornis during blood-feeding. Parasit. Vectors 2018, 11, 91. [Google Scholar] [CrossRef] [Green Version]
- Vieira, P.; Rajewsky, K. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 1988, 18, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.; Rajewsky, K. The bulk of endogenously produced IgG2a is eliminated from the serum of adult C57BL/6 mice with a half-life of 6–8 days. Eur. J. Immunol. 1986, 16, 871–874. [Google Scholar] [CrossRef]
- Albas, A.; Fontolan, O.l.; Pardo, P.E.; Bremer Neto, H.; Sartori, A. Interval between first dose and booster affected antibody production in cattle vaccinated against rabies. J. Venom. Anim. Toxins Incl. Trop. Dis. 2006, 12, 476–486. [Google Scholar] [CrossRef]
- Castiglione, F.; Mantile, F.; De Berardinis, P.; Prisco, A. How the interval between prime and boost injection affects the immune response in a computational model of the immune system. Comput. Math. Methods Med. 2012, 2012, 842329. [Google Scholar] [CrossRef] [Green Version]
- Leo, O.; Cunningham, A.; Stern, P.L. Vaccine immunology. In Understanding Modern Vaccines; Garcon, N., Stern, P., Cunningham, T., Stanberry, L., Eds.; Elsevier: Philadelphia, PA, USA, 2011; Volume 25, p. 29. [Google Scholar]
- Wang, H.L. Revisiting the adult vaccination schedule for tetanus and diphtheria. AJN Am. J. Nurs. 2016, 116, 15. [Google Scholar] [CrossRef]
- Ni, Y.H.; Huang, L.M.; Chang, M.H.; Yen, C.J.; Lu, C.Y.; You, S.L.; Kao, J.H.; Lin, Y.C.; Chen, H.L.; Hsu, H.J.; et al. Two decades of universal hepatitis B vaccination in Taiwan: Impact and implication for future strategies. Gastroenterology 2007, 132, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.; Montero, C.; Sánchez, D.; Pérez, D.; Valdés, M.; Alfonso, A.; Joglar, M.; Machado, H.; Rodríguez, E.; Méndez, L.; et al. Two initial vaccinations with the Bm86-based Gavacplus vaccine against Rhipicephalus (Boophilus) microplus induce similar reproductive suppression to three initial vaccinations under production conditions. BMC Vet. Res. 2010, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Mathers, A.R.; Larregina, A.T. Professional antigen-presenting cells of the skin. Immunol. Res. 2006, 36, 127–136. [Google Scholar] [CrossRef]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Bridon, J.M.; Fayette, J.; Barthélémy, C.; Banchereau, J.; Caux, C.; Brière, F. Dendritic cells directly modulate B cell growth and differentiation. J. Leukoc. Biol. 1999, 66, 224–230. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Röcken, M.; Ghoreschi, K. Cutaneous immunology: Basics and new concepts. Semin. Immunopathol. 2016, 38, 3–10. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef]
- Wangmo, K.; Laven, R.; Cliquet, F.; Wasniewski, M.; Yang, A. Comparison of antibody titres between intradermal and intramuscular rabies vaccination using inactivated vaccine in cattle in Bhutan. PLoS ONE 2019, 14, e0209946. [Google Scholar] [CrossRef] [Green Version]
- Wahl, M.; Hermodsson, S. Intradermal, subcutaneous or intramuscular administration of hepatitis B vaccine: Side effects and antibody response. Scand. J. Infec. Dis. 1987, 19, 617–621. [Google Scholar] [CrossRef]
- Ols, S.; Yang, L.; Thompson, E.A.; Pushparaj, P.; Tran, K.; Liang, F.; Lin, A.; Eriksson, B.; Hedestam, G.B.K.; Wyatt, R.T.; et al. Route of vaccine administration alters antigen trafficking but not innate or adaptive immunity. Cell Rep. 2020, 30, 3964–3971. [Google Scholar] [CrossRef]
- Smith, G.N.; Griffiths, B.; Mollison, D.; Mollison, P.L. Uptake of IgG after intramuscular and subcutaneous injection. Lancet 1972, 1, 1208–1212. [Google Scholar] [CrossRef]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Hopf, S.; Garner-Spitzer, E.; Hofer, M.; Kundi, M.; Wiedermann, U. Comparable immune responsiveness but increased reactogenicity after subcutaneous versus intramuscular administration of tick borne encephalitis (TBE) vaccine. Vaccine 2016, 34, 2027–2034. [Google Scholar] [CrossRef]
- Frazer, I.H.; Jones, B.; Dimitrakakis, M.; Mackay, I.R. Intramuscular versus low-dose intradermal hepatitis B vaccine. Assessment by humoral and cellular immune response to hepatitis B surface antigen. Med. J. Aust. 1987, 146, 242–245. [Google Scholar] [CrossRef]
- McCluskie, M.J.; Weeratna, R.D.; Payette, P.J.; Davis, H.L. Parenteral and mucosal prime-boost immunization strategies in mice with hepatitis B surface antigen and CpG DNA. FEMS Immunol. Med. Microbiol. 2002, 32, 179–185. [Google Scholar] [CrossRef]
- Contreras, M.; Kasaija, P.D.; Merino, O.; de la Cruz-Hernandez, N.I.; Gortazar, C.; de la Fuente, J. Oral vaccination with a formulation combining Rhipicephalus microplus subolesin with heat inactivated Mycobacterium bovis reduces tick infestations in cattle. Front. Cell. Infect. Microbiol. 2019, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Squier, C.A. The permeability of oral mucosa. Crit. Rev. Oral. Biol. Med. 1991, 2, 13–32. [Google Scholar] [CrossRef]
- Otczyk, D.C.; Cripps, A.W. Mucosal immunization: Realistic alternative. Hum. Vaccin. 2010, 6, 978–1006. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Chaphalkar, S.R. Vaccine Adjuvants: The current necessity of life. Shiraz E-Med. J. 2015, 16, e28061. [Google Scholar] [CrossRef]
- Uddowla, S.; Freytag, L.C.; Clements, J.D. Effect of adjuvants and route of immunizations on the immune response to recombinant plague antigens. Vaccine 2007, 25, 7984–7993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radaelli, E.; Santagostino, S.F.; Sellers, R.S.; Brayton, C.F. Immune relevant and immune deficient mice: Options and opportunities in translational research. ILAR J. 2018, 59, 211–246. [Google Scholar] [CrossRef]
- Turner, R.J.; Held, S.D.; Hirst, J.E.; Billinghurst, G.; Wootton, R.J. An immunological assessment of group-housed rabbits. Lab. Anim. 1997, 31, 362–372. [Google Scholar] [CrossRef]
- Tabor, A.E.; Ali, A.; Rehman, G.; Rocha Garcia, G.; Zangirolamo, A.F.; Malardo, T.; Jonsson, N.N. Cattle tick Rhipicephalus microplus host interface: A Review of resistant and susceptible host responses. Front. Cell. Infect. Microbiol. 2017, 7, 506. [Google Scholar] [CrossRef] [Green Version]
- Bakheit, M.A.; Latif, A.A. The innate resistance of Kenana cattle to tropical theileriosis (Theileria annulata infection) in the Sudan. Ann. N. Y. Acad. Sci. 2002, 969, 159–163. [Google Scholar] [CrossRef]
- Glass, E.J.; Jensen, K. Resistance and susceptibility to a protozoan parasite of cattle-gene expression differences in macrophages from different breeds of cattle. Vet. Immunol. Immunopathol. 2007, 120, 20–30. [Google Scholar] [CrossRef]
- Piper, E.K.; Jonsson, N.N.; Gondro, C.; Lew-Tabor, A.E.; Moolhuijzen, P.; Vance, M.E.; Jackson, L.A. Immunological profiles of Bos taurus and Bos indicus cattle infested with the cattle tick, Rhipicephalus (Boophilus) microplus. Clin. Vaccine Immunol. 2009, 16, 1074–1086. [Google Scholar] [CrossRef] [Green Version]
- Plowright, W. The duration of immunity in cattle following inoculation of rinderpest cell culture vaccine. Am. J. Hyg. 1984, 92, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, C.E.; Kim, C.; Weyand, C.M.; Goronzy, J.J. Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol. 2020, 145, 1309–1321. [Google Scholar] [CrossRef]
- Uspensky, I.; Ioffe-Uspensky, I. The relationship between engorged female weight and egg number in ixodid ticks: A biological interpretation of linear regression parameters. Acarologia 1999, 40, 9–17. [Google Scholar]
- Pereira, M. Daily mean number of eggs laid by the southern cattle tick (Acari: Ixodidae) compared with mean egg mass weight. J. Econ. Entomol. 1998, 91, 153–158. [Google Scholar] [CrossRef]
- Do Amaral, M.A.; Prata, M.C.; Daemon, E.; Furlong, J. Biological parameters of cattle ticks fed on rabbits. Rev. Bras. Parasitol. Vet. 2012, 21, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Bailey, K.P. Notes on the rearing of Rhipicephalus appendiculatus and their infection with Theileria parva for experimental transmission. Bull. Epizoot. Dis. Afr. 1960, 8, 33–64. [Google Scholar]
- Wang, H.; Paesen, G.C.; Nuttall, P.A.; Barbour, A.G. Male ticks help their mates to feed. Nature 1998, 391, 753–754. [Google Scholar] [CrossRef]
- Stjernberg, L.; Berglund, J. Detecting ticks on light versus dark clothing. Scand. J. Infec. Dis. 2005, 37, 361–364. [Google Scholar] [CrossRef]
- Senbill, H.; Hazarika, L.K.; Baruah, A.; Borah, D.K.; Bhattacharyya, B.; Rahman, S. Life cycle of the southern cattle tick, Rhipicephalus (Boophilus) microplus Canestrini 1888 (Acari: Ixodidae) under laboratory conditions. Syst. Appl. Acarol. 2018, 23, 1169–1179. [Google Scholar] [CrossRef]
- Scheerlinck, J.-P.Y. The immune system of sheep and goats. Encycl. Immunobiol. 2016, 1, 526–531. [Google Scholar] [CrossRef]
- Castro, J.; Cunningham, M.; Dolan, T.; Dransfield, R.; Newson, R.; Young, A. Effects on cattle of artificial infestations with the tick Rhipicephalus appendiculatus. Parasitology 1985, 90, 21–33. [Google Scholar] [CrossRef]
- Merino, O.; Alberdi, P.; Pérez de la Lastra, J.M.; de la Fuente, J. Tick vaccines and the control of tick-borne pathogens. Front. Cell Infect. Microbiol. 2013, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Valle, M.R.; Guerrero, F.D. Anti-tick vaccines in the omics era. Front. Biosci. 2018, 10, 122–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente, J.; Rodríguez, M.; Redondo, M.; Montero, C.; García-García, J.C.; Méndez, L.; Serrano, E.; Valdés, M.; Enriquez, A.; Canales, M.; et al. Field studies and cost-effectiveness analysis of vaccination with Gavac against the cattle tick Boophilus microplus. Vaccine 1998, 16, 366–373. [Google Scholar] [CrossRef]
- De la Fuente, J.; Rodríguez, M.; Montero, C.; Redondo, M.; García-García, J.C.; Méndez, L.; Serrano, E.; Valdés, M.; Enríquez, A.; Canales, M.; et al. Vaccination against ticks (Boophilus spp.): The experience with the Bm86-based vaccine Gavac(TM). Genet. Anal. Biomol. Eng. 1999, 15, 143–148. [Google Scholar] [CrossRef]
- De la Fuente, J.; Almazán, C.; Canales, M.; Pérez de la Lastra, J.M.; Kocan, K.M.; Willadsen, P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health Res. Rev. 2007, 8, 23–28. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Pound, J.; Davey, R. Acaricides for Controlling Ticks on Cattle and the Problem of Acaricide Resistance; Bowman, A., Nuttall, P., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 408–423. [Google Scholar]
- Fernandez-Ruvalcaba, M.; Preciado-De-La Torre, F.; Cruz-Vazquez, C.; Garcia-Vazquez, Z. Anti-tick effects of Melinis minutiflora and Andropogon gayanus grasses on plots experimentally infested with Boophilus microplus larvae. Exp. Appl. Acarol. 2004, 32, 293–299. [Google Scholar] [CrossRef]
- Ferguson, H.M.; Ng’habi, K.R.; Walder, T.; Kadungula, D.; Moore, S.J.; Lyimo, I.; Russell, T.L.; Urassa, H.; Mshinda, H.; Killeen, G.F.; et al. Establishment of a large semi-field system for experimental study of African malaria vector ecology and control in Tanzania. Malar. J. 2008, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- Ng’habi, K.R.; Mwasheshi, D.; Knols, B.G.; Ferguson, H.M. Establishment of a self-propagating population of the African malaria vector Anopheles arabiensis under semi-field conditions. Malar. J. 2010, 9, 356. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.C.; Roa, E.J.; Romero, N.T. Anti-tick grasses as the basis for developing practical tropical tick control packages. Trop. Anim. Health Prod. 1978, 10, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Furlong, J. Infesting power of Boophilus microplus larvae (acari: Ixodidae) in Melinis minutiflora, Brachiaria decumbens e Brachiaria mutica pastures. Cienc. Rural. 1998, 28, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Designed by the Skyline Consults Ltd, Uganda. Available online: https://www.skylinearchitectsug.com/ (accessed on 20 January 2021).
- Halloran, M.E.; Struchiner, C.J. Study designs for dependent happenings. Epidemiology 1991, 2, 331–338. [Google Scholar] [CrossRef]
- John, T.J.; Samuel, R. Herd immunity and herd effect: New insights and definitions. Eur. J. Epidemiol. 2000, 16, 601–606. [Google Scholar] [CrossRef]
- Francis, M.J. A Veterinary vaccine development process map to assist in the development of new vaccines. Vaccine 2020, 38, 4512–4515. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndawula, C., Jr. From Bench to Field: A Guide to Formulating and Evaluating Anti-Tick Vaccines Delving beyond Efficacy to Effectiveness. Vaccines 2021, 9, 1185. https://doi.org/10.3390/vaccines9101185
Ndawula C Jr. From Bench to Field: A Guide to Formulating and Evaluating Anti-Tick Vaccines Delving beyond Efficacy to Effectiveness. Vaccines. 2021; 9(10):1185. https://doi.org/10.3390/vaccines9101185
Chicago/Turabian StyleNdawula, Charles, Jr. 2021. "From Bench to Field: A Guide to Formulating and Evaluating Anti-Tick Vaccines Delving beyond Efficacy to Effectiveness" Vaccines 9, no. 10: 1185. https://doi.org/10.3390/vaccines9101185