Degradomics-Based Analysis of Tetanus Toxoids as a Quality Control Assay
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
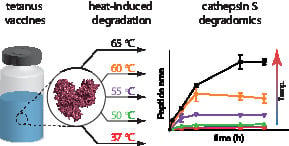
3.1. Preparation of Aberrant Toxoids
3.2. Degradomics Analysis
3.3. Evaluation of Heat-Treated Toxoids by Traditional Techniques
3.4. Al(OH)3 and AlPO4 Adsorbed Tetanus Toxoid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Plotkin, S.A.; Orenstein, W.A.; Offit, P.A.; Roper, M.H.; Wassilak, S.G.F.; Scobie, H.M.; Ridpath, A.D. Tetanus Toxoid. In Vaccines, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2018; p. 1061. [Google Scholar]
- WHO. Production and Control of Tetanus Vaccine: Module III: Principles of Tetanus Vaccine Production. WHO/VSQ/94.4. Available online: http://whqlibdoc.who.int/hq/1994/WHO_VSQ_GEN_94.4.pdf (accessed on 26 March 2020).
- Council of Europe. European Pharmacopoeia 10.0. In Tetanus Vaccine (Adsorbed); Council of Europe: Strasbourg, France, 2020; pp. 1042–1043. [Google Scholar]
- Council of Europe. European Pharmacopoeia 8.0. In Insulin Aspart; Council of Europe: Strasbourg, France, 2014; pp. 2485–2486. [Google Scholar]
- Council of Europe. European Pharmacopoeia 8.0. In Monoclonal Antibodies for Human Use; Council of Europe: Strasbourg, France, 2014; pp. 753–755. [Google Scholar]
- Council of Europe. European Pharmacopoeia 10.0. In Assay of Tetanus Vaccine (Adsorbed); Council of Europe: Strasbourg, France, 2020; pp. 275–278. [Google Scholar]
- Bruysters, M.W.; Schiffelers, M.-J.; Hoonakker, M.; Jungbaeck, C.; Ragan, I.; Rommel, E.; Van Der Stappen, T.; Viviani, L.; Hessel, E.V.; Akkermans, A.M.; et al. Drivers and barriers in the consistency approach for vaccine batch release testing: Report of an international workshop. Biologicals 2017, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, F.; Hendriksen, C.; Buchheit, K.H.; Chapsal, J.M.; Halder, M.; Lambrigts, D.; Redhead, K.; Rommel, E.; Scharton-Kersten, T.; Sesardic, T.; et al. The vaccines consistency approach project: An EPAA initiative. Pharmeuropa Bio Sci. Notes 2015, 2015, 30–56. [Google Scholar]
- De Mattia, F.; Chapsal, J.-M.; Descamps, J.; Halder, M.; Jarrett, N.; Kross, I.; Mortiaux, F.; Ponsar, C.; Redhead, K.; McKelvie, J.; et al. The consistency approach for quality control of vaccines—A strategy to improve quality control and implement 3Rs. Biologicals 2011, 39, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Carmicle, S.; Dai, G.; Steede, N.K.; Landry, S.J. Proteolytic Sensitivity and Helper T-cell Epitope Immunodominance Associated with the Mobile Loop in Hsp10s. J. Biol. Chem. 2002, 277, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.; Hartman, I.Z.; Poore, B.; Boronina, T.; Cole, R.N.; Song, N.; Ciudad, M.T.; Caspi, R.R.; Jaraquemada, D.; Sadegh-Nasseri, S. Divergent paths for the selection of immunodominant epitopes from distinct antigenic sources. Nat. Commun. 2014, 5, 5369. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Jürets, A.; Wallner, M.; Briza, P.; Ruzek, S.; Hainzl, S.; Pichler, U.; Kitzmüller, C.; Bohle, B.; Huber, C.G.; et al. Assessing Protein Immunogenicity with a Dendritic Cell Line-Derived Endolysosomal Degradome. PLoS ONE 2011, 6, e17278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delamarre, L.; Couture, R.; Mellman, I.; Trombetta, E.S. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J. Exp. Med. 2006, 203, 2049–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, Y.; Freier, R.; Scheiblhofer, S.; Thalhamer, T.; Mayr, M.; Briza, P.; Grutsch, S.; Ahammer, L.; Fuchs, J.E.; Wallnoefer, H.G.; et al. Fold stability during endolysosomal acidification is a key factor for allergenicity and immunogenicity of the major birch pollen allergen. J. Allergy Clin. Immunol. 2016, 137, 1525–1534. [Google Scholar] [CrossRef] [Green Version]
- Freier, R.; Dall, E.; Brandstetter, H. Protease recognition sites in Bet v 1a are cryptic, explaining its slow processing relevant to its allergenicity. Sci. Rep. 2015, 5, 12707. [Google Scholar] [CrossRef] [Green Version]
- Kitzmüller, C.; Wallner, M.; Deifl, S.; Mutschlechner, S.; Walterskirchen, C.; Zlabinger, G.J.; Ferreira, F.; Bohle, B. A hypoallergenic variant of the major birch pollen allergen shows distinct characteristics in antigen processing and T-cell activation. Allergy 2012, 67, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Michiels, T.J.M.; Meiring, H.D.; Jiskoot, W.; Kersten, G.F.A.; Metz, B. Formaldehyde treatment of proteins enhances proteolytic degradation by the endo-lysosomal protease cathepsin S. Sci. Rep. 2020, 10, 11535. [Google Scholar] [CrossRef] [PubMed]
- Doekhie, A.; Dattani, R.; Chen, Y.-C.; Yang, Y.; Smith, A.; Silve, A.P.; Koumanov, F.; Wells, S.A.; Edler, K.J.; Marchbank, K.J.; et al. Ensilicated tetanus antigen retains immunogenicity: In vivo study and time-resolved SAXS characterization. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Milstein, J.B. Temperature Sensitivity of Vaccines; WHO/IVB/06.10; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Cohen, H.; Van Ramshorst, J.D.; Tasman, A. Consistency in potency assay of tetanus toxoid in mice. Bull. World Health Organ. 1959, 20, 1133–1150. [Google Scholar]
- Meiring, H.D.; Van Der Heeft, E.; Hove, G.J.T.; De Jong, A.P.J.M. Nanoscale LC-MS(n): Technical design and applications to peptide and protein analysis. J. Sep. Sci. 2002, 25, 557–568. [Google Scholar] [CrossRef]
- Metz, B.; Kersten, G.F.A.; Hoogerhout, P.; Brugghe, H.F.; Timmermans, H.A.M.; De Jong, A.; Meiring, H.; Hove, J.T.; Hennink, W.E.; Crommelin, D.J.A.; et al. Identification of Formaldehyde-induced Modifications in Proteins. J. Biol. Chem. 2004, 279, 6235–6243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, B.; Jiskoot, W.; Hennink, W.E.; Crommelin, D.J.; Kersten, G.F. Physicochemical and immunochemical techniques predict the quality of diphtheria toxoid vaccines. Vaccine 2003, 22, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Catak, S.; Monard, G.; Aviyente, V.; Ruiz-López, M.F. Deamidation of Asparagine Residues: Direct Hydrolysis versus Succinimide-Mediated Deamidation Mechanisms. J. Phys. Chem. A 2009, 113, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, H.; Wiederanders, B. Cathepsin S and related lysosomal endopeptidases. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1994; Volume 244, pp. 500–511. [Google Scholar]








| Name | Source | Experiment | Temperature Exposure (Duration) |
|---|---|---|---|
| TTd-A1.1 | In-house | Peptide selection and evaluation of kinetics | 4, 50, 55, 60, 65 °C, (2 days) 37 °C, (30 days) |
| TTd-A1.2 | In-house | Quantification with isotopically labeled standard | 4, 37, 45, 50, 55, 60, 65 °C, (2 days) |
| TTd-B1.1 | Manufacturer B | Quantification with isotopically labeled standard | 4, 37, 45, 50, 55, 60, 65 °C, (2 days) |
| TTd-C1 | Manufacturer C | Quantification with isotopically labeled standard | 4, 37, 45, 50, 55, 60, 65 °C, (2 days) |
| TTd-B1.2 | Manufacturer B | Evaluation of batch consistency | 4 °C |
| TTd-B1PC | Manufacturer B | Evaluation of batch consistency positive control | 55 °C, (2 days) |
| TTd-B2 | Manufacturer B | Evaluation of batch consistency | 4 °C |
| TTd-B3 | Manufacturer B | Evaluation of batch consistency | 4 °C |
| TTd-B4 | Manufacturer B | Evaluation of batch consistency | 4 °C |
| TTd-B5 | Manufacturer B | Evaluation of batch consistency | 4 °C |
| DTd | Manufacturer B Diphtheria toxoid | Evaluation of batch consistency negative control | 4 °C |
| TTd-B1.3 | Manufacturer B | Adsorbed toxoids control (no adjuvant added) | 4, 37, 45, 50, 55, 60, 65 °C, (2 days) |
| TTd-B1.4 | Manufacturer B | Adsorbed toxoids Al(OH)3 adsorbed | 4, 37, 45, 50, 55, 60, 65 °C, (2 days) |
| TTd-B1.5 | Manufacturer B | Adsorbed toxoids AlPO4 adsorbed | 4, 37, 45, 50, 55, 60, 65 °C, (2 days) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michiels, T.J.M.; Tilstra, W.; Hamzink, M.R.J.; de Ridder, J.W.; Danial, M.; Meiring, H.D.; Kersten, G.F.A.; Jiskoot, W.; Metz, B. Degradomics-Based Analysis of Tetanus Toxoids as a Quality Control Assay. Vaccines 2020, 8, 712. https://doi.org/10.3390/vaccines8040712
Michiels TJM, Tilstra W, Hamzink MRJ, de Ridder JW, Danial M, Meiring HD, Kersten GFA, Jiskoot W, Metz B. Degradomics-Based Analysis of Tetanus Toxoids as a Quality Control Assay. Vaccines. 2020; 8(4):712. https://doi.org/10.3390/vaccines8040712
Chicago/Turabian StyleMichiels, Thomas J. M., Wichard Tilstra, Martin R. J. Hamzink, Justin W. de Ridder, Maarten Danial, Hugo D. Meiring, Gideon F. A. Kersten, Wim Jiskoot, and Bernard Metz. 2020. "Degradomics-Based Analysis of Tetanus Toxoids as a Quality Control Assay" Vaccines 8, no. 4: 712. https://doi.org/10.3390/vaccines8040712