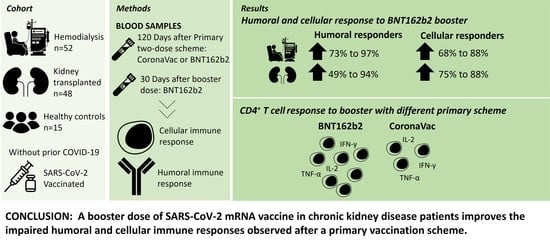
A Third Dose of SARS-CoV-2 mRNA Vaccine Improves Immune Response in Chronic Kidney Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol and Participants
2.2. Cellular Immune Response
2.3. Humoral Response
2.4. Statistical Analysis
3. Results
3.1. Humoral and Cellular Immune Response Increases Significantly after a Third Dose in CKD Patients
3.2. Booster Vaccination Elicits Robust Polyfunctional CD4+ T Cell Responses in CKD Patients
3.3. CD4+ and CD8+ IFN-γ Memory T Cell Response Increases in CKD Patients after a BNT162b2 Booster
3.4. Immunosuppressive Drugs and Vaccine Response in KT Patients
3.5. Outcome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ERA-EDTA Council; ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: A call to action by the ERA-EDTA. Nephrol. Dial. Transpl. 2021, 36, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Syed-Ahmed, M.; Narayanan, M. Immune Dysfunction and Risk of Infection in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Pefaur, J.; Toro, L.; Lorca, E.; Torres, R.; FUTAC Team. Impact of a National Multicentric Strategy to Support Kidney Transplant Patients During the COVID-19 Pandemic in Latin America: FUTAC Team Creation and Activities. Transplantation 2022, 106, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Penna, J.P.; Toro, L.; Rosati, P.; Badilla, X.; Ardiles, L.; Rocca, X.; Valenzuela, M.; Mur, P.; Boltansky, A.; Diaz, C.; et al. COVID-19 infection in chilean renal transplanted patients: Incidence and clinical outcomes. Colaborative multicentric study. Kidney Int. Rep. 2021, 6, S331. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Torres, R.; Toro, L.; Sanhueza, M.E.; Lorca, E.; Ortiz, M.; Pefaur, J.; Clavero, R.; Machuca, E.; Gonzalez, F.; Herrera, P.; et al. Clinical Efficacy of SARS-CoV-2 Vaccination in Hemodialysis Patients. Kidney Int. Rep. 2022, 7, 2176–2185. [Google Scholar] [CrossRef]
- Dulovic, A.; Strengert, M.; Ramos, G.M.; Becker, M.; Griesbaum, J.; Junker, D.; Lürken, K.; Beigel, A.; Wrenger, E.; Lonnemann, G.; et al. Diminishing Immune Responses against Variants of Concern in Dialysis Patients 4 Months after SARS-CoV-2 mRNA Vaccination. Emerg. Infect. Dis. 2022, 28, 743–750. [Google Scholar] [CrossRef]
- Affeldt, P.; Koehler, F.C.; Brensing, K.A.; Adam, V.; Burian, J.; Butt, L.; Gies, M.; Grundmann, F.; Hinrichs, S.; Johannis, W.; et al. Immune Responses to SARS-CoV-2 Infection and Vaccination in Dialysis Patients and Kidney Transplant Recipients. Microorganisms 2021, 10, 4. [Google Scholar] [CrossRef]
- Bertrand, D.; Hamzaoui, M.; Lemée, V.; Lamulle, J.; Hanoy, M.; Laurent, C.; Lebourg, L.; Etienne, I.; Lemoine, M.; Le Roy, F.; et al. Antibody and T Cell Response to SARS-CoV-2 Messenger RNA BNT162b2 Vaccine in Kidney Transplant Recipients and Hemodialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 2147–2152. [Google Scholar] [CrossRef]
- Kolb, T.; Fischer, S.; Müller, L.; Lubke, N.; Hillebrandt, J.; Andrée, M.; Schmitz, M.; Schmidt, C.; Küçükköylü, S.; Koster, L.; et al. Impaired Immune Response to SARS-CoV-2 Vaccination in Dialysis Patients and in Kidney Transplant Recipients. Kidney360 2021, 2, 1491–1498. [Google Scholar] [CrossRef]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.-M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.-L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, J.; Siepmann, T.; Lindner, T.; Karger, C.; Schwöbel, J.; Anders, L.; Faulhaber-Walter, R.; Schewe, J.; Martin, H.; Schirutschke, H.; et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: A prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg. Health Eur. 2021, 9, 100178. [Google Scholar] [CrossRef] [PubMed]
- Dheir, H.; Tocoglu, A.; Toptan, H.; Pinar, M.; Demirci, T.; Koroglu, M.; Yaylaci, S.; Genc, A.B.; Genc, A.C.; Firat, N.; et al. Short and mid-term SARS-CoV-2 antibody response after inactivated COVID-19 vaccine in hemodialysis and kidney transplant patients. J. Med. Virol. 2022, 94, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, I.Z.; Oster, Y.; Tzukert, K.; Alster, T.; Bader, R.; Israeli, R.; Asayag, H.; Aharon, M.; Burstein, I.; Pri-Chen, H.; et al. Impact of tozinameran (BNT162b2) mRNA vaccine on kidney transplant and chronic dialysis patients: 3-5 months follow-up. J. Nephrol. 2022, 35, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Bruminhent, J.; Setthaudom, C.; Chaumdee, P.; Boongird, S.; Kiertiburanakul, S.; Malathum, K.; Nongnuch, A.; Phuphuakrat, A.; Jirasiritham, S.; Janphram, C.; et al. SARS-CoV-2-specific humoral and cell-mediated immune responses after immunization with inactivated COVID-19 vaccine in kidney transplant recipients (CVIM 1 study). Am. J. Transplant. 2022, 22, 813–822. [Google Scholar] [CrossRef]
- Sattler, A.; Schrezenmeier, E.; Weber, U.A.; Potekhin, A.; Bachmann, F.; Straub-Hohenbleicher, H.; Budde, K.; Storz, E.; Proß, V.; Bergmann, Y.; et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Investig. 2021, 131, e150175. [Google Scholar] [CrossRef]
- Gonzalez-Perez, M.; Montes-Casado, M.; Conde, P.; Cervera, I.; Baranda, J.; Berges-Buxeda, M.J.; Perez-Olmeda, M.; Sanchez-Tarjuelo, R.; Utrero-Rico, A.; Lozano-Ojalvo, D.; et al. Development of Potent Cellular and Humoral Immune Responses in Long-Term Hemodialysis Patients After 1273-mRNA SARS-CoV-2 Vaccination. Front. Immunol. 2022, 13, 845882. [Google Scholar] [CrossRef]
- Broseta, J.J.; Rodríguez-Espinosa, D.; Rodríguez, N.; Mosquera, M.D.M.; Marcos, M.Á.; Egri, N.; Pascal, M.; Soruco, E.; Bedini, J.L.; Bayés, B.; et al. Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients. Am. J. Kidney Dis. 2021, 78, 571–581. [Google Scholar] [CrossRef]
- Chavarot, N.; Ouedrani, A.; Marion, O.; Leruez-Ville, M.; Vilain, E.; Baaziz, M.; Del Bello, A.; Burger, C.; Sberro-Soussan, R.; Martinez, F.; et al. Poor Anti-SARS-CoV-2 Humoral and T-cell Responses After 2 Injections of mRNA Vaccine in Kidney Transplant Recipients Treated with Belatacept. Transplantation 2021, 105, e94–e95. [Google Scholar] [CrossRef]
- Zhang, R.; Shin, B.-H.; Gadsden, T.-A.M.; Petrosyan, A.; Vo, A.; Ammerman, N.; Sethi, S.; Huang, E.; Peng, A.; Najjar, R.; et al. Assessment of humoral and cellular immune responses to SARS-CoV-2 vaccination (BNT162b2) in immunocompromised renal allograft recipients. Transpl. Infect. Dis. 2022, 24, e13813. [Google Scholar] [CrossRef] [PubMed]
- Panizo, N.; Albert, E.; Giménez-Civera, E.; Puchades, M.J.; D’Marco, L.; Gandía-Salmerón, L.; Giménez, E.; Torre, I.; Sancho, A.; Gavela, E.; et al. Dynamics of SARS-CoV-2-Spike-reactive antibody and T-cell responses in chronic kidney disease patients within 3 months after COVID-19 full vaccination. Clin. Kidney J. 2022, 15, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Stefanski, A.-L.; Potekhin, A.; Staub-Hohenbleicher, H.; Choi, M.; Bachmann, F.; Proβ, V.; Hammett, C.; Schrezenmeier, H.; et al. B and T Cell Responses after a Third Dose of SARS-CoV-2 Vaccine in Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 3027–3033. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, T.H.; Seibert, F.S.; Anft, M.; Blazquez-Navarro, A.; Skrzypczyk, S.; Zgoura, P.; Meister, T.L.; Pfaender, S.; Stumpf, J.; Hugo, C.; et al. A third vaccine dose substantially improves humoral and cellular SARS-CoV-2 immunity in renal transplant recipients with primary humoral nonresponse. Kidney Int. 2021, 100, 1135–1136. [Google Scholar] [CrossRef]
- Grievink, H.W.; Luisman, T.; Kluft, C.; Moerland, M.; Malone, K.E. Comparison of Three Isolation Techniques for Human Peripheral Blood Mononuclear Cells: Cell Recovery and Viability, Population Composition, and Cell Functionality. Biopreserv. Biobank 2016, 14, 410–415. [Google Scholar] [CrossRef]
- Rey-Jurado, E.; Espinosa, Y.; Astudillo, C.; Cortés, L.J.; Hormazabal, J.; Noguera, L.P.; Cofré, F.; Piñera, C.; González, R.; Bataszew, A.; et al. Deep immunophenotyping reveals biomarkers of MIS-C in a Latin American cohort. J. Allergy Clin. Immunol. 2022, 150, 1074–1085. e11. [Google Scholar] [CrossRef]
- Roederer, M.; Nozzi, J.L.; Nason, M.C. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011, 79, 167–174. [Google Scholar] [CrossRef]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef]
- Rose, R.; Neumann, F.; Grobe, O.; Lorentz, T.; Fickenscher, H.; Krumbholz, A. Humoral immune response after different SARS-CoV-2 vaccination regimens. BMC Med. 2022, 20, 31. [Google Scholar] [CrossRef]
- Bertoletti, A.; Le Bert, N.; Qui, M.; Tan, A.T. SARS-CoV-2-specific T cells in infection and vaccination. Cell. Mol. Immunol. 2021, 18, 2307–2312. [Google Scholar] [CrossRef]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008, 8, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Brlek, P.; Matišić, V.; Molnar, V.; Vrdoljak, K.; Zadro, R.; Parčina, M. Cellular Immunity-The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination. Vaccines 2022, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, J.; Siepmann, T.; Schwöbel, J.; Glombig, G.; Paliege, A.; Steglich, A.; Gembardt, F.; Kessel, F.; Kröger, H.; Arndt, P.; et al. MMF/MPA Is the Main Mediator of a Delayed Humoral Response With Reduced Antibody Decline in Kidney Transplant Recipients After SARS-CoV-2 mRNA Vaccination. Front. Med. 2022, 9, 928542. [Google Scholar] [CrossRef] [PubMed]
- Kantauskaite, M.; Müller, L.; Kolb, T.; Fischer, S.; Hillebrandt, J.; Ivens, K.; Andree, M.; Luedde, T.; Orth, H.M.; Adams, O.; et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am. J. Transplant. 2022, 22, 634–639. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.-B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020, 183, 158–168. e14. [Google Scholar] [CrossRef]
- Amorim, L.V.P.; Cristelli, M.P.; Viana, L.A.; Dreige, Y.C.; Requião-Moura, L.R.; Nakamura, M.R.; Foresto, R.D.; Medina-Pestana, J.; Tedesco-Silva, H. Immunogenicity, Reactogenicity, and Reinfection after 2 Doses of the Inactivated Whole-virion CoronaVac Vaccine in Kidney Transplant Recipients Convalescents from COVID-19. Transplantation 2022, 106, 853–861. [Google Scholar] [CrossRef]
- Dib, M.; Le Corre, N.; Ortiz, C.; García, D.; Ferrés, M.; Martinez-Valdebenito, C.; Ruiz-Tagle, C.; Ojeda, M.J.; Espinoza, M.A.; Jara, A.; et al. SARS-CoV-2 vaccine booster in solid organ transplant recipients previously immunised with inactivated versus mRNA vaccines: A prospective cohort study. Lancet Reg. Health Am. 2022, 16, 100371. [Google Scholar] [CrossRef]
- Balcells, M.E.; Le Corre, N.; Durán, J.; Ceballos, M.E.; Vizcaya, C.; Mondaca, S.; Dib, M.; Rabagliati, R.; Sarmiento, M.; Burgos, P.I.; et al. Reduced Immune Response to Inactivated Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine in a Cohort of Immunocompromised Patients in Chile. Clin. Infect. Dis. 2022, 75, e594–e602. [Google Scholar] [CrossRef]
- Medina-Pestana, J.; Covas, D.T.; Viana, L.A.; Dreige, Y.C.; Nakamura, M.R.; Lucena, E.F.; Requião-Moura, L.R.; Fortaleza, C.M.C.B.; Foresto, R.D.; Tedesco-Silva, H.; et al. Inactivated Whole-virus Vaccine Triggers Low Response Against SARS-CoV-2 Infection Among Renal Transplant Patients: Prospective Phase 4 Study Results. Transplantation 2022, 106, 853–861. [Google Scholar] [CrossRef]
- Eren Sadioğlu, R.; Demir, E.; Evren, E.; Aktar, M.; Şafak, S.; Artan, A.S.; Meşe, S.; Ağaçfidan, A.; Çınar, G.; Önel, M.; et al. Antibody response to two doses of inactivated SARS-CoV-2 vaccine (CoronaVac) in kidney transplant recipients. Transpl. Infect. Dis. 2021, 23, e13740. [Google Scholar] [CrossRef]
- Clavero, R.; Parra-Lucares, A.; Méndez-Valdés, G.; Villa, E.; Bravo, K.; Mondaca, E.; Aranda, J.; Brignardello, R.; Gajardo, C.; Ordenes, A.; et al. Humoral Immune Response of BNT162b2 and CoronaVac Vaccinations in Hemodialysis Patients: A Multicenter Prospective Cohort. Vaccines 2022, 10, 1542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-M.; Shi, R.; Wang, P.; He, J.; Chen, Y.; Feng, Y.-T.; Pan, H.-F.; Wang, D.-G. Early Humoral Responses of Hemodialysis Patients After Inactivated SARS-CoV-2 Vaccination. J. Inflamm. Res. 2022, 15, 3467–3475. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Dhrolia, M.; Qureshi, H.; Qureshi, R.; Nasir, K.; Ahmad, A. Comparison of COVID-19 Inactivated Virus Vaccine Immunogenicity Between Healthy Individuals and Patients on Hemodialysis: A Single-Center Study from Pakistan. Cureus 2022, 14, e24153. [Google Scholar] [CrossRef] [PubMed]
- Bruminhent, J.; Setthaudom, C.; Kitpermkiat, R.; Kiertiburanakul, S.; Malathum, K.; Assanatham, M.; Nongnuch, A.; Phuphuakrat, A.; Chaumdee, P.; Janphram, C.; et al. Immunogenicity of ChAdOx1 nCoV-19 vaccine after a two-dose inactivated SARS-CoV-2 vaccination of dialysis patients and kidney transplant recipients. Sci. Rep. 2022, 12, 3587. [Google Scholar] [CrossRef]
- Di Fusco, M.; Lin, J.; Vaghela, S.; Lingohr-Smith, M.; Nguyen, J.L.; Scassellati Sforzolini, T.; Judy, J.; Cane, A.; Moran, M.M. COVID-19 vaccine effectiveness among immunocompromised populations: A targeted literature review of real-world studies. Expert. Rev. Vaccines 2022, 21, 435–451. [Google Scholar] [CrossRef]
- Hardgrave, H.; Wells, A.; Nigh, J.; Klutts, G.; Krinock, D.; Osborn, T.; Bhusal, S.; Rude, M.K.; Burdine, L.; Giorgakis, E. COVID-19 Mortality in Vaccinated vs. Unvaccinated Liver & Kidney Transplant Recipients: A Single-Center United States Propensity Score Matching Study on Historical Data. Vaccines 2022, 10, 1921. [Google Scholar]
- Sanhueza, M.E.; San Martin, P.; Brantes, L.; Caro, S.; Carrasco, G.; Machuca, E. Efficacy of vaccination against the SARS-CoV-2 virus in patients with chronic kidney disease on hemodialysis. Hum. Vaccin. Immunother. 2023, 19, 2173904. [Google Scholar] [CrossRef]
- Betts, M.R.; Nason, M.C.; West, S.M.; De Rosa, S.C.; Migueles, S.A.; Abraham, J.; Lederman, M.M.; Benito, J.M.; Goepfert, P.A.; Connors, M. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006, 107, 4781–4789. [Google Scholar] [CrossRef]
- Price, D.A.; Brenchley, J.M.; Ruff, L.E.; Betts, M.R.; Hill, B.J.; Roederer, M.; Koup, R.A.; Migueles, S.A.; Gostick, E.; Wooldridge, L.; et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 2005, 202, 1349–1361. [Google Scholar] [CrossRef]
- Benning, L.; Morath, C.; Bartenschlager, M.; Kim, H.; Reineke, M.; Beimler, J.; Buylaert, M.; Nusshag, C.; Kälble, F.; Reichel, P.; et al. Neutralizing antibody response against the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants after a third mRNA SARS-CoV-2 vaccine dose in kidney transplant recipients. Am. J. Transplant. 2022, 22, 1873–1883. [Google Scholar] [CrossRef]
- Bouwmans, P.; Messchendorp, A.L.; Imhof, C.; Sanders, J.-S.F.; Hilbrands, L.B.; Reinders, M.E.J.; Vart, P.; Bemelman, F.J.; Abrahams, A.C.; van den Dorpel, R.M.A.; et al. Impact of immunosuppressive treatment and type of SARS-CoV-2 vaccine on antibody levels after three vaccinations in patients with chronic kidney disease or kidney replacement therapy. Clin. Kidney J. 2023, 16, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Karnell, J.L.; Karnell, F.G., 3rd; Stephens, G.L.; Rajan, B.; Morehouse, C.; Li, Y.; Swerdlow, B.; Wilson, M.; Goldbach-Mansky, R.; Groves, C.; et al. Mycophenolic acid differentially impacts B cell function depending on the stage of differentiation. J. Immunol. 2011, 187, 3603–3612. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Jens, A.; Stefanski, A.-L.; Hammett, C.; Osmanodja, B.; Koch, N.; Zukunft, B.; Beck, J.; Oellerich, M.; et al. Temporary antimetabolite treatment hold boosts SARS-CoV-2 vaccination-specific humoral and cellular immunity in kidney transplant recipients. JCI Insight 2022, 7, e157836. [Google Scholar] [CrossRef]
- Lee, W.-C.; Hung, H.-C.; Lee, J.-C.; Huang, C.-G.; Huang, P.-W.; Gu, P.-W.; Wang, Y.-C.; Cheng, C.-H.; Wu, T.-H.; Lee, C.-F.; et al. Adjustment of Immunosuppressants to Facilitate Anti-COVID-19 Antibody Production after mRNA Vaccination in Liver Transplant Recipients. Viruses 2023, 15, 678. [Google Scholar] [CrossRef] [PubMed]
- Housset, P.; Kubab, S.; Hanafi, L.; Pardon, A.; Vittoz, N.; Bozman, D.-F.; Caudwell, V.; Faucon, A.-L. Humoral response after a fourth “booster” dose of a Coronavirus disease 2019 vaccine following a 3-dose regimen of mRNA-based vaccination in dialysis patients. Kidney Int. 2022, 101, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Masset, C.; Benotmane, I.; Dantal, J.; Garandeau, C.; Gauthier-Vargas, G.; Cantarovich, D.; Meurette, A.; Giral, M.; Caillard, S.; Blancho, G. A fourth SARS-CoV-2 mRNA vaccine in strictly seronegative kidney transplant recipients. Kidney Int. 2022, 101, 825–826. [Google Scholar] [CrossRef]
- Ministerio de Salud de Chile. Calendario de Vacunación Masiva Contra COVID-19. 2022. Available online: https://www.minsal.cl/calendario-de-vacunacion-masiva-contra-covid-19/ (accessed on 13 December 2022).
- Gao, Y.; Cai, C.; Grifoni, A.; Müller, T.R.; Niessl, J.; Olofsson, A.; Humbert, M.; Hansson, L.; Österborg, A.; Bergman, P.; et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 2022, 28, 472–476. [Google Scholar] [CrossRef]






| Variables | Hemodialysis (n = 48) | Kidney Transplant (n = 52) |
|---|---|---|
| Age (mean years ± SD) | 68.3 ± 13.9 | 53.7 ± 12.7 |
| Gender | ||
| Female | 12 (25.0%) | 17 (32.7%) |
| Male | 36 (75.0%) | 35 (67.3%) |
| BMI (mean kg/m2 ± SD) | 26.2 ± 3.5 | 26.8 ± 4.6 |
| Primary vaccination schedule | ||
| CoronaVac | 42 (87.5%) | 35 (67.3%) |
| BNT162b2 | 6 (12.5%) | 17 (32.7%) |
| CKD etiology | ||
| Mellitus diabetes | 15 (31.2%) | 7 (13.5%) |
| Unknown | 20 (41.7%) | 13 (25.0%) |
| Glomerular | 8 (16.7%) | 18 (34.6%) |
| Congenital/genetic | 4 (8.3%) | 11 (21.1%) |
| Others | 1 (2.1%) | 3 (5.8%) |
| Time after kidney replacement therapies:Hemodialysis or transplant (mean days ± SD) | 1915 ± 1786 | 2125.6 ± 6007.3 |
| Immunosuppression | None of the patients received pharmacological immunosuppression. | |
| FK+MPA+PND | 36 (69.2%) | |
| FK+Aza+PND | 4 (7.7%) | |
| FK+Eve+PND | 7 (13.5%) | |
| FK+Rapa+PND | 1 (1.9%) | |
| CSA+Aza+PND | 2 (3.8%) | |
| Belatacept | 2 (3.8%) | |
| Charlson Score (mean ± SD) | 6 ± 2.7 | 4 ± 2.1 |
| Death | 2 (4.2%) | 1 (1.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poli, M.C.; Vial, C.; Rey-Jurado, E.; González, N.; Cortés, L.J.; Hormazabal, J.; Ramírez-Riffo, C.; de la Cruz, J.; Ulloa, C. A Third Dose of SARS-CoV-2 mRNA Vaccine Improves Immune Response in Chronic Kidney Disease Patients. Vaccines 2023, 11, 1012. https://doi.org/10.3390/vaccines11051012
Poli MC, Vial C, Rey-Jurado E, González N, Cortés LJ, Hormazabal J, Ramírez-Riffo C, de la Cruz J, Ulloa C. A Third Dose of SARS-CoV-2 mRNA Vaccine Improves Immune Response in Chronic Kidney Disease Patients. Vaccines. 2023; 11(5):1012. https://doi.org/10.3390/vaccines11051012
Chicago/Turabian StylePoli, Maria Cecilia, Cecilia Vial, Emma Rey-Jurado, Natalia González, Lina Jimena Cortés, Juan Hormazabal, Carolina Ramírez-Riffo, Javiera de la Cruz, and Camilo Ulloa. 2023. "A Third Dose of SARS-CoV-2 mRNA Vaccine Improves Immune Response in Chronic Kidney Disease Patients" Vaccines 11, no. 5: 1012. https://doi.org/10.3390/vaccines11051012