Testable Candidate Immune Correlates of Protection for Porcine Reproductive and Respiratory Syndrome Virus Vaccination
Abstract
:1. Correlates of Protective Immunity
- i.
- Large challenge doses may overwhelm vaccine-induced immunity and confuse the identification of correlates,
- ii.
- The mechanism of protection is not necessarily the mechanism of recovery from infection,
- iii.
- Most vaccines available today act through antibodies; however, the immune system is redundant and may protect through multiple mechanisms (paraphrased),
- iv.
- Memory induced by vaccination may be crucial to protection, particularly in long-incubation diseases,
- v.
- Correlates may vary according to individual characteristics, such as age, gender, and major histocompatibility complex,
- vi.
- It is important to define protection against what; what is the prevention objective (paraphrased).
2. The Problem of PRRSV
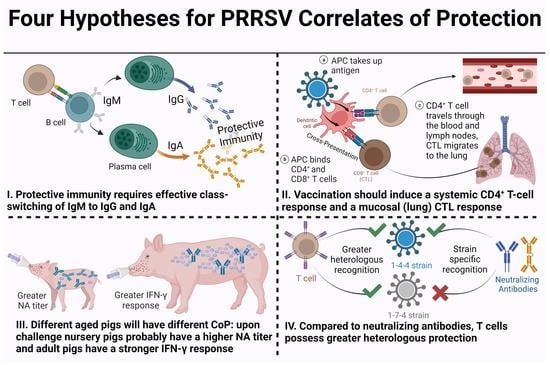
3. Plotkin’s Principles of Correlates of Protection Applied to PRRSV
- I.
- Effective class-switching to systemic IgG and mucosal IgA neutralizing antibodies is required for protective immunity.
- II.
- Vaccination should induce virus-specific peripheral blood CD4+ T-cell proliferation and interferon gamma (IFN-γ) production with central memory and effector memory phenotypes; cytotoxic T-lymphocytes (CTL) proliferation and IFN-γ production with a CCR7- phenotype that should migrate to the lung.
- III.
- Pigs are vaccinated prior to breeding and at weaning: nursery, finishing, and adult pigs will have different CoP. Nursery pigs will generally have a higher neutralizing antibodies’ titer upon challenge than adult pigs: adult pigs should have a stronger IFN-γ response than nursery pigs.
- IV.
- Neutralizing antibodies provide protection and are rather strain specific; T cells confer disease prevention/reduction and possess greater heterologous recognition.
3.1. Effective Class-Switching to Systemic IgG and Mucosal IgA Neutralizing Antibodies Is Required for Protective Immunity
3.2. Vaccination Should Induce Systemic CD4+ T-cell Memory and Lung CD8+ T-cell Memory
- Vaccination should induce virus-specific peripheral blood CD4+ T-cell proliferation and IFN-γ production with central memory and effector memory phenotypes.
- Proliferating and IFN-γ producing CTLs should indicate a CCR7- phenotype that allows them to migrate to the site of infection—the lung or the reproductive tract.
3.3. Nursery Pigs Will Generally Have a Higher NA Titer upon Challenge Than Adult Pigs: Adult Pigs Should Have a Stronger IFN-γ Response Than Nursery Pigs
3.4. Neutralizing Antibodies Provide Protection and Are Strain Specific; T cells Confer Disease Prevention/Reduction and Possess Greater Heterologous Recognition
4. New Approaches for Immune Correlates of Protection Evaluation
- I.
- Serum NA either after vaccination or challenge correlate with reduced viremia and PRRSV pathology.
- II.
- Cell-mediated immunity is quantifiable with IFN-γ production in specific T-cell phenotypes, and as expected in viral infection: induction of the T-cell response (T helper and CTL) results in clearance of infection and improved performance.
- III.
- Age greatly affects the measured response to PRRSV: nursery-age pigs and gilts/sows demonstrate different characteristics in their immune response.
- IV.
- Serum NAs induced from vaccination or infection are more likely to be strain specific than T cells, which enable an improved response across PRRSV strains.
| Strain | Pig Age | Vaccination | IFN-γ Response | Serum NA | Challenge | Viremia/Symptoms | IFN-γ Response | Serum NA | Reference |
|---|---|---|---|---|---|---|---|---|---|
| PRRSV-2 | N | IN KV-2 +adj on KV-2 | KV-2 + adj INC at 28 dpv | KV-2 + adj INC at 28 dpv (also nasal IgA) | HO WT-2 at 28 dpv | KV-2 + adj (Lung WT-2 DEC) | All vacc INC in CD4+, CD8+ phenotypes | All vacc INC; Adj-vacc INC highest | [120] |
| PRRSV-2 | N | KV-2 | NE | NE | HO WT-2 | Vacc DEC, 7–10 dpc | Vacc INC 10 dpc in CD4+, and CD8+ | Titer 10 dpc in Vacc | [121] |
| PRRSV-2 | N | IM MLV-2 | ND | ND (anti-PRRSV nasal IgA induced for vacc) | HE WT-2 28 dpv | DEC in Vacc | Vacc/unvacc HE INC 14 dpc; Vacc CD4+ and CD8+ INC over unvacc for NC174 & 1-4-2 | INC in Vacc against ¾ strains (not NC174) | [129] |
| PRRSV-2 | N | IM and ID MLV-2 and ID or IM PCV2 | HO and HE SCs INC in vacc at 28 dpv | NE | HE WT-2 | DEC in all vacc | HO & HE SCs in INC in vacc 7 dpc; unvacc detectable 7 dpc | NE | [179] |
| PRRSV-1 | G/S | MLV-1 and second KV-1 3w before farrow | NE | INC in double Vacc and their piglets as measured weaning | CSE on two farms | Double vacc offspring DEC PRRSV+ and lower mortality | NE | NE | [75] |
| PRRSV-2 | N | N/A | N/A | N/A | IN WT-2 or IM MLV-2 | All viremic | HO and HE in WT at 28 dpi | HO at 14 or 21 dpi in WT and HE at 28 or 42 dpi in WT; MLV HO only and low | [67,130] |
| PRRSV-2 | N, G | G series of MLV-2 and KV-2 during gestation | CD4+ largest IFN-γ producers 7d before farrowing | MLV-2 + KV-2 had WT NA; MLV only not HE | N, HO or HE WT-2 at 14d of age | Lung lesions DEC in MLV-2 + KV-2 | No diff between vacc; IFN-γ prod shifts with age | MD NA detectable and prot in MLV-2 + KV-2 | [76] |
| PRRSV-1 | N | MLV-1 at 1 day-old | NE | NE | HE WT-1 at 28 dpv | DEC in VAC | INC SCs in Vacc and positively correlated with DEC viremia | NE | [85] |
| PRRSV-1 | N | MLV-1 in conjunction with swine influenza A | NE | NE | HE WT-1 at 28 dpv | Co-infection at vacc did not affect viremia/symptoms | INC SCs in vacc at 15 dpc | Detected at 15dpc across challenged | [180] |
| PRRSV-1 | F | IM MLV-1 (3 dose series); two strains | NE | HO beginning at 21 dpv through 70 dpv (3rd vacc) | HE WT-1 various strains at 70 dpv | No clinical signs; vacc lower viremia | NE | Vacc with broadly NA achieved sterilizing immunity in 5/8 chall groups | [117] |
| PRRSV-2 | N | IM MLV-2 | ND | ND | HE WT-2 at 33 dpv with swine influenza | DEC in VAC, unless co-infected | Vacc induced CD8 prod: no difference post challenge | ND | [161] |
| PRRSV-1 | N | IM KV-1 + adj + booster | Vacc induced Th, CTLs, Tm 2 wp booster in two trts | NE | HO WT-1 at 50 dpv | No sig diff between vacc/unvacc trts | Unvacc INC in Th, CTLs, Tm post challenge | NE | [160] |
| PRRSV-2 | N | DNA GP5-mosaic or GP5-WT (IM & ID) | mRNA INC HO and HE at 35 dpv for GP5-mosaic; HO for WT | HO and HE at 35 dpv for GP5-mosaic; HO for WT | WT-2 IN and IM at 35 dpv with HO or HE strain | GP5-mosaic DEC viremia and pathology for HO and HE | NE | NE | [159] |
| PRRSV-1 PRRSV-2 | N | MLV-1 (IM v. ID) | HO at 28–35 dpv | HO and HE at 21–35 dpv | HE WT-2, WT-1 | Vacc DEC and ID vacc had lower viremia than IM | HE SCs appear in vacc 7 dpc | HO and HE continues through 35 dpc | [108] |
| PRRSV-2 | F | IM MLV-2 | NE | 118 dpv for 2/3 HE strains (not 1-7-4) | HE WT-2 | Viremia only detected in 1-7-4 challenged | NE | Vacc induced HE NA after challenge | [50] |
| PRRSV-2 | G | MLV-2 | Vacc INC SCs 42–135 dpv | Vacc INC 42–135 dpv | HE WT-2 | Vacc INC reproductive performance | Vacc INC SCs | Vacc INC NA after challenge | [80] |
| PRRSV-2 | N | N/A | N/A | N/A | WT-2 | Peak at 10 dpc | IFN-γ+ cells early in local response peak at 21 dpc | Appeared 28 dpc | [150] |
| PRRSV-2 | N | CPD of WT | HO/HE 28 dpv | HO only at 28 dpv | CPD vs. HE WT | CPD lower | HO/HE 14 dpi | HO only 14 dpi | [113] |
| PRRSV-1 PRRSV-2 | mice | KV w/mAb adj in mice | HE at 28 dpv | mAb cross-reactive | N/A | N/A | N/A | N/A | [181] |
| PRRSV-1 PRRSV-2 | G/S | MLV-1 or MLV-2 | 2 HE w/HO higher; 1 HO only 21 dpv | Both HE/w HO higher 21 dpv | Both HE WT-1&2 | MLV-2 reduced 1&2 | HE w/HO higher; 2 more SCs | HE w/HO higher | [118] |
| PRRSV-1 | N | MLV or MLV w/DNA | MLV w/DNA higher SCs to peptides 13 dpv | NE | N/A | N/A | N/A | N/A | [131] |
| PRRSV-1 | N | 4 types (2 KV, 2 MLV) | Positive for all vaccines 21 dpv | MLV higher 21 dpv | HE WT-1 | Virus shed lower in vacc | Positive to include unvacc/challenged | Inactivated higher 21 dpv | [109] |
| PRRSV-1 | N,F | MLV IN or IM | SCs INC thru 125 dpv | Appeared 56 dpv thru 125 dpv | HE WT-1 | Reduced in vacc | IN INC post-chall; IM DEC 10 dpi | 3-fold INC vacc 10 dpi | [83] |
| PRRSV-1 PRRSV-2 | G/S | MLV-1 & MLV-2 | HE 21 dpv | HE 21 dpv | Both HE WT-1&2 | Vacc higher RP | SCs higher 7 & 21 dpi | Titer higher 7 & 21 dpi | [169] |
| PRRSV-2 | G/S | MLV-2 | HO 70 dpv | HO 70 dpv | CSE | Vacc no viremia/higher RP | DEC at farrow | DEC at farrow | [119] |
| PRRSV-2 | N | N/A | N/A | N/A | cDNA clone, FL12 | N/A | SCs highest 63 & 77 dpi; genetic similarity did not affect SC with type 2 PRRSV | HO NA titers at 63 & 77 dpi; limited HE titers | [122] |
| PRRSV-2 | N | MLV-2 | Peaked 21 dpv | ND | HE WT-2 isolates | Vacc DEC | Rose rapidly thru 14 dpi | ND | [111] |
| PRRSV-1 PRRSV-2 | N | WT-1 or WT-2 | Low-level HO & HE post-chall | ND | WT-1 or WT-2 (HO or HE) | HO second chall; DEC | HO second chall; rapid rise 3 dpc | HO second chall; rapid rise 7 dpc | [171] |
| PRRSV-1 | N | MLV-1 | NE | NE | CSE | Vacc INC morbidity & survival | Vacc INC 7 wpv thru 16 wpv; unvacc higher Treg activation | NE | [133] |
| PRRSV-2 | N | MLV-2 or MLV-2 + adj | Higher SC 14 dpv MLV + adj | NE | N/A | N/A | N/A | N/A | [157] |
| PRRSV-1 PRRSV-2 | N | MLV-2 | NE | NE | HE WT-1 | Vacc reduced | Higher vacc SC 7–21 dpc | NE | [182] |
| PRRSV-1 PRRSV-2 | G/S | MLV-2 | SCs INC to 84 dpv; DEC 121 dpv HO virus | ND | HE WT-1 or WT-2 | HO chall prot; HE chall semi-prot | SC to HO virus in vacc highest; all HO SC INC after infection | HO chall highest NA; reduced for HE chall | [165] |
| PRRSV-2 | N | MLV-2 | NE | ND | HO or HE WT-2 | MLV + HO-2 chall prot | MLV + WT challenge had highest SCs | HO present 14 dpi | [166] |
| PRRSV-2 | N | MLV-2 | SCs INC 7, 14 dpv | ND | HE WT-2 | Vacc DEC | SCs INC rapidly 7–14 dpc in vacc | ND | [156] |
| PRRSV-1 | N | MLV-1 (IM vs. ID vs. adj only) | SCs to HO & HE at 21, 35 dpv | ND | CSE | Similar across trts | SCs INC to HO & HE at 35 dpi in vacc | Titers present after CSE in vacc | [155] |
| PRRSV-1 | N | MLV-1, PCV2 or both | SCs INC after MLV-1 | NE | CSE | Similar across trts | SCs INC all trts after CSE | NE | [183] |
| PRRSV-1 | N | 2x WT-1 isolates | WT-1(1) more SCs than WT-1(2) | WT-1(2) more NA than WT-1(1) | Challenge w/either WT-1 isolate | WT-1(2) no viremia; WT-1(1) low viremia | WT-1(1) more SCs than WT-1(2) | WT-1(1) more NA than WT-1(2) | [142] |
| PRRSV-2 | N | N/A | N/A | N/A | WT-2 | Viremic 7 dpi; 50% 56 dpi | Detected 28 dpi; peaked 42 dpi; steady 56–193 dpi | NA appear 42 dpi; peak 70 dpi; remain 193 dpi | [167] |
| PRRSV-1 | G/S | MLV-1, KV-1 | SCs background for both | ND | HE WT-1 | KV viremic; MLV prot | Low, more SCs in MLV than background | KV rose 10 dpi: MLV background | [173] |
| PRRSV-1 | F | N/A | N/A | N/A | WT-1 | Clear w/in 7–14 dpi | SCs INC 14–70 dpi | NA appear 56 dpi in 60% | [145] |
| PRRSV-2 | N | MLV-2 | SCs peaked at 28 dpv, then DEC | NE | HE WT-2 cocktail | WT-2 persists in lymph nodes 67 dpi | No correlation SCs with virus presence in tissues 19 or 67 dpi | NE | [184] |
| PRRSV-2 | N, F | Recombi-nant component in BCG | PRRSVSCs ND | Detectable 60 dpi | HO WT-2 | Vacc DEC | PRRSV SCs ND | NA not INC 7 dpi | [185] |
| PRRSV-2 | F | MLV-2 + adj | SCs peaked 4 wpi | Minimal thru 8 wpv | HE WT-2 | Vacc lower/no viremia at 4, 7 dpc | SCs INC after chall thru 14 dpc | Vacc NA INC 14 dpc to vacc & chall | [168] |
| PRRSV-2 | F | MLV-2, KV-2, PRV, or MLV-2 + adj | MLV-2 vac SCs INC thru 8 wpv; less than PRV | NA detectable at 8 wpv; less than PRV | N/A | N/A | N/A | N/A | [153] |
| PRRSV-2 | N-A | N/A | N/A | N/A | WT-2 | Persisted 3 wpi | SCs maintained 5–12 mpi | NA appear with SCs; DEC post-viremia | [186] |
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Text Abbreviations
References
- Plotkin, S.A.; Orenstein, W.A.; Offit, P.A. Plotkin’s Vaccines, 7th ed.; Elsevier: Philadelphia, PA, USA, 2018; p. xxi. 1691p. [Google Scholar]
- Plotkin, S.A.; Gilbert, P.B. Nomenclature for immune correlates of protection after vaccination. Clin. Infect. Dis. 2012, 54, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [Green Version]
- Plotkin, S.A. Complex correlates of protection after vaccination. Clin. Infect. Dis. 2013, 56, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Updates on immunologic correlates of vaccine-induced protection. Vaccine 2019, 38, 2250–2257. [Google Scholar] [CrossRef]
- Murtaugh, M.P.; Genzow, M. Immunological solutions for treatment and prevention of porcine reproductive and respiratory syndrome (PRRS). Vaccine 2011, 29, 8192–8204. [Google Scholar] [CrossRef]
- Chae, C. Commercial PRRS Modified-Live Virus Vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Brinton, M.A.; Gulyaeva, A.A.; Balasuriya, U.B.R.; Dunowska, M.; Faaberg, K.S.; Goldberg, T.; Leung, F.C.C.; Nauwynck, H.J.; Snijder, E.J.; Stadejek, T.; et al. ICTV Virus Taxonomy Profile: Arteriviridae 2021. J. Gen. Virol. 2021, 102, 001632. [Google Scholar] [CrossRef]
- Murtaugh, M.P.; Faaberg, K.S.; Laber, J.; Elam, M.; Kapur, V. Genetic variation in the PRRS virus. Adv. Exp. Med. Biol. 1998, 440, 787–794. [Google Scholar]
- Forsberg, R. Divergence time of porcine reproductive and respiratory syndrome virus subtypes. Mol. Biol. Evol. 2005, 22, 2131–2134. [Google Scholar] [CrossRef]
- Ruedas-Torres, I.; Rodriguez-Gomez, I.M.; Sanchez-Carvajal, J.M.; Larenas-Munoz, F.; Pallares, F.J.; Carrasco, L.; Gomez-Laguna, J. The jigsaw of PRRSV virulence. Vet. Microbiol. 2021, 260, 109168. [Google Scholar] [CrossRef]
- Opriessnig, T.; Mattei, A.A.; Karuppannan, A.K.; Halbur, P.G. Future perspectives on swine viral vaccines: Where are we headed? Porcine Health Manag. 2021, 7, 1. [Google Scholar] [CrossRef]
- Rahe, M.C.; Murtaugh, M.P. Mechanisms of Adaptive Immunity to Porcine Reproductive and Respiratory Syndrome Virus. Viruses 2017, 9, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Key, N.; McBride, W.D. The Changing Economics of US Hog Production. USDA-ERS Econ. Res. Rep. 2007, 52, 5–11. [Google Scholar]
- Lee, K.; Polson, D.; Lowe, E.; Main, R.; Holtkamp, D.; Martinez-Lopez, B. Unraveling the contact patterns and network structure of pig shipments in the United States and its association with porcine reproductive and respiratory syndrome virus (PRRSV) outbreaks. Prev. Vet. Med. 2017, 138, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.K.; Revie, C.W.; Hurnik, D.; Poljak, Z.; Sanchez, J. Analysis of Swine Movement in Four Canadian Regions: Network Structure and Implications for Disease Spread. Transbound. Emerg. Dis. 2016, 63, e14–e26. [Google Scholar] [CrossRef]
- Buttner, K.; Krieter, J.; Traulsen, A.; Traulsen, I. Static network analysis of a pork supply chain in Northern Germany-Characterisation of the potential spread of infectious diseases via animal movements. Prev. Vet. Med. 2013, 110, 418–428. [Google Scholar] [CrossRef]
- Torremorell, M.; Moore, C.; Christianson, W.T. Establishment of a herd negative for porcine reproductive and respiratory syndrome virus (PRRSV) from PRRSV positive sources. J. Swine Health Prod. 2002, 10, 153–160. [Google Scholar]
- Corzo, C.A.; Mondaca, E.; Wayne, S.; Torremorell, M.; Dee, S.; Davies, P.; Morrison, R.B. Control and elimination of porcine reproductive and respiratory syndrome virus. Virus Res. 2010, 154, 185–192. [Google Scholar] [CrossRef]
- Schaefer, N.; Morrison, R. Effect on total pigs weaned of herd closure for elimination of porcine reproductive and respiratory syndrome virus. J. Swine Health Prod. 2007, 15, 152–155. [Google Scholar]
- Amadori, M.; Listorti, V.; Razzuoli, E. Reappraisal of PRRS Immune Control Strategies: The Way Forward. Pathogens 2021, 10, 1073. [Google Scholar] [CrossRef]
- Mortensen, S.; Stryhn, H.; Sogaard, R.; Boklund, A.; Stark, K.D.; Christensen, J.; Willeberg, P. Risk factors for infection of sow herds with porcine reproductive and respiratory syndrome (PRRS) virus. Prev. Vet. Med. 2002, 53, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Brockmeier, S.L.; Lager, K.M. Experimental airborne transmission of porcine reproductive and respiratory syndrome virus and Bordetella bronchiseptica. Vet. Microbiol. 2002, 89, 267–275. [Google Scholar] [CrossRef]
- Arruda, A.G.; Tousignant, S.; Sanhueza, J.; Vilalta, C.; Poljak, Z.; Torremorell, M.; Alonso, C.; Corzo, C.A. Aerosol Detection and Transmission of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): What Is the Evidence, and What Are the Knowledge Gaps? Viruses 2019, 11, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pileri, E.; Mateu, E. Review on the transmission porcine reproductive and respiratory syndrome virus between pigs and farms and impact on vaccination. Vet. Res. 2016, 47, 108. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.; Gray, G.C. The Mandate for a Global “One Health” Approach to Antimicrobial Resistance Surveillance. Am. J. Trop. Med. Hyg. 2019, 100, 227–228. [Google Scholar] [CrossRef] [Green Version]
- Tassinari, E.; Duffy, G.; Bawn, M.; Burgess, C.M.; McCabe, E.M.; Lawlor, P.G.; Gardiner, G.; Kingsley, R.A. Microevolution of antimicrobial resistance and biofilm formation of Salmonella Typhimurium during persistence on pig farms. Sci. Rep. 2019, 9, 8832. [Google Scholar] [CrossRef] [Green Version]
- Holtkamp, D.J.; Kliebenstein, J.B.; Neumann, E.J.; Zimmerman, J.J.; Rotto, H.; Yoder, T.K.; Wang, C.; Yeske, P.; Mowrer CHaley, C. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J. Swine Health Prod. 2013, 21, 72–84. [Google Scholar]
- Zimmerman, J.J. Diseases of Swine, 10th ed.; Wiley-Blackwell: Chichester, UK, 2012; p. xxiii. 983p. [Google Scholar]
- Butler, J.E.; Lager, K.M.; Golde, W.; Faaberg, K.S.; Sinkora, M.; Loving, C.; Zhang, Y.I. Porcine reproductive and respiratory syndrome (PRRS): An immune dysregulatory pandemic. Immunol. Res. 2014, 59, 81–108. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Andino, R. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 2010, 6, e1001005. [Google Scholar] [CrossRef] [Green Version]
- Botner, A.; Strandbygaard, B.; Sorensen, K.J.; Have, P.; Madsen, K.G.; Madsen, E.S.; Alexandersen, S. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet. Rec. 1997, 141, 497–499. [Google Scholar] [CrossRef]
- Wang, C.; Wu, B.; Amer, S.; Luo, J.; Zhang, H.; Guo, Y.; Dong, G.; Zhao, B.; He, H. Phylogenetic analysis and molecular characteristics of seven variant Chinese field isolates of PRRSV. BMC Microbiol. 2010, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Madsen, K.G.; Hansen, C.M.; Madsen, E.S.; Strandbygaard, B.; Botner, A.; Sorensen, K.J. Sequence analysis of porcine reproductive and respiratory syndrome virus of the American type collected from Danish swine herds. Arch. Virol. 1998, 143, 1683–1700. [Google Scholar] [CrossRef]
- Shi, M.; Holmes, E.C.; Brar, M.S.; Leung, F.C. Recombination is associated with an outbreak of novel highly pathogenic porcine reproductive and respiratory syndrome viruses in China. J. Virol. 2013, 87, 10904–10907. [Google Scholar] [CrossRef] [Green Version]
- Brar, M.S.; Shi, M.; Hui, R.K.; Leung, F.C. Genomic evolution of porcine reproductive and respiratory syndrome virus (PRRSV) isolates revealed by deep sequencing. PLoS ONE 2014, 9, e88807. [Google Scholar] [CrossRef]
- Kappes, M.A.; Faaberg, K.S. PRRSV structure, replication and recombination: Origin of phenotype and genotype diversity. Virology 2015, 479–480, 475–486. [Google Scholar] [CrossRef] [Green Version]
- Wenhui, L.; Zhongyan, W.; Guanqun, Z.; Zhili, L.; JingYun, M.; Qingmei, X.; Baoli, S.; Yingzuo, B. Complete genome sequence of a novel variant porcine reproductive and respiratory syndrome virus (PRRSV) strain: Evidence for recombination between vaccine and wild-type PRRSV strains. J. Virol. 2012, 86, 9543. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Lam, T.T.; Hon, C.C.; Murtaugh, M.P.; Davies, P.R.; Hui, R.K.; Li, J.; Wong, L.T.; Yip, C.W.; Jiang, J.W.; et al. Phylogeny-based evolutionary, demographical, and geographical dissection of North American type 2 porcine reproductive and respiratory syndrome viruses. J. Virol. 2010, 84, 8700–8711. [Google Scholar] [CrossRef] [Green Version]
- Martin-Valls, G.E.; Kvisgaard, L.K.; Tello, M.; Darwich, L.; Cortey, M.; Burgara-Estrella, A.J.; Hernandez, J.; Larsen, L.E.; Mateu, E. Analysis of ORF5 and full-length genome sequences of porcine reproductive and respiratory syndrome virus isolates of genotypes 1 and 2 retrieved worldwide provides evidence that recombination is a common phenomenon and may produce mosaic isolates. J. Virol. 2014, 88, 3170–3181. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Lam, T.T.; Hon, C.C.; Hui, R.K.; Faaberg, K.S.; Wennblom, T.; Murtaugh, M.P.; Stadejek, T.; Leung, F.C. Molecular epidemiology of PRRSV: A phylogenetic perspective. Virus Res. 2010, 154, 7–17. [Google Scholar] [CrossRef]
- Xie, C.; Ha, Z.; Nan, F.; Zhang, Y.; Zhang, H.; Li, J.; Zhang, P.; Han, J.; Zhuang, X.; Zhang, J.; et al. Characterization of porcine reproductive and respiratory syndrome virus (ORF5 RFLP 1-7-4 viruses) in northern China. Microb. Pathog. 2020, 140, 103941. [Google Scholar] [CrossRef]
- Paploski, I.A.D.; Corzo, C.; Rovira, A.; Murtaugh, M.P.; Sanhueza, J.M.; Vilalta, C.; Schroeder, D.C.; VanderWaal, K. Temporal Dynamics of Co-circulating Lineages of Porcine Reproductive and Respiratory Syndrome Virus. Front. Microbiol. 2019, 10, 2486. [Google Scholar] [CrossRef] [Green Version]
- Lambert, M.E.; Delisle, B.; Arsenault, J.; Poljak, Z.; D’Allaire, S. Positioning Quebec ORF5 sequences of porcine reproductive and respiratory syndrome virus (PRRSV) within Canada and worldwide diversity. Infect. Genet. Evol. 2019, 74, 103999. [Google Scholar] [CrossRef]
- Van Geelen, A.G.M.; Anderson, T.K.; Lager, K.M.; Das, P.B.; Otis, N.J.; Montiel, N.A.; Miller, L.C.; Kulshreshtha, V.; Buckley, A.C.; Brockmeier, S.L.; et al. Porcine reproductive and respiratory disease virus: Evolution and recombination yields distinct ORF5 RFLP 1-7-4 viruses with individual pathogenicity. Virology 2018, 513, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Crisci, E.; Fraile, L.; Montoya, M. Cellular Innate Immunity against PRRSV and Swine Influenza Viruses. Vet. Sci. 2019, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Bordet, E.; Maisonnasse, P.; Renson, P.; Bouguyon, E.; Crisci, E.; Tiret, M.; Descamps, D.; Bernelin-Cottet, C.; Urien, C.; Lefevre, F.; et al. Porcine Alveolar Macrophage-like cells are pro-inflammatory Pulmonary Intravascular Macrophages that produce large titers of Porcine Reproductive and Respiratory Syndrome Virus. Sci. Rep. 2018, 8, 10172. [Google Scholar] [CrossRef] [Green Version]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Pathogenesis and Interaction with the Immune System. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Mengeling, W.L.; Lager, K.M.; Vorwald, A.C. Clinical consequences of exposing pregnant gilts to strains of porcine reproductive and respiratory syndrome (PRRS) virus isolated from field cases of “atypical” PRRS. Am. J. Vet. Res. 1998, 59, 1540–1544. [Google Scholar] [PubMed]
- Rahe, M.C.; Dvorak, C.M.T.; Patterson, A.; Roof, M.; Murtaugh, M.P. The PRRSV-Specific Memory B Cell Response Is Long-Lived in Blood and Is Boosted During Live Virus Re-exposure. Front. Immunol. 2020, 11, 247. [Google Scholar] [CrossRef] [Green Version]
- Loving, C.L.; Osorio, F.A.; Murtaugh, M.P.; Zuckermann, F.A. Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus. Vet. Immunol. Immunopathol. 2015, 167, 1–14. [Google Scholar] [CrossRef]
- Cao, Q.M.; Tian, D.; Heffron, C.L.; Subramaniam, S.; Opriessnig, T.; Foss, D.L.; Calvert, J.G.; Meng, X.J. Cytotoxic T lymphocyte epitopes identified from a contemporary strain of porcine reproductive and respiratory syndrome virus enhance CD4+CD8+ T, CD8+ T, and gammadelta T cell responses. Virology 2019, 538, 35–44. [Google Scholar] [CrossRef]
- Montaner-Tarbes, S.; Del Portillo, H.A.; Montoya, M.; Fraile, L. Key Gaps in the Knowledge of the Porcine Respiratory Reproductive Syndrome Virus (PRRSV). Front. Vet. Sci. 2019, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Ma, L.; Yang, M.; Wu, W.; Feng, W.; Chen, Z. The Function of the PRRSV–Host Interactions and Their Effects on Viral Replication and Propagation in Antiviral Strategies. Vaccines 2021, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Y.; Feng, W. Porcine Reproductive and Respiratory Syndrome Virus: Immune Escape and Application of Reverse Genetics in Attenuated Live Vaccine Development. Vaccines 2021, 9, 480. [Google Scholar] [CrossRef]
- Sun, H.; Pattnaik, A.K.; Osorio, F.A.; Vu, H.L.X. Identification of viral genes associated with the interferon-inducing phenotype of a synthetic porcine reproductive and respiratory syndrome virus strain. Virology 2016, 499, 313–321. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Cai, X.; Zhou, E.M.; Zimmerman, J.J. Effects of PRRSV Infection on the Porcine Thymus. Trends Microbiol. 2020, 28, 212–223. [Google Scholar] [CrossRef]
- Käser, T.; Gerner, W.; Hammer, S.E.; Patzl, M.; Saalmüller, A. Phenotypic and functional characterisation of porcine CD4(+)CD25(high) regulatory T cells. Vet. Immunol. Immunopathol. 2008, 122, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Käser, T.; Gerner, W.; Saalmüller, A. Porcine regulatory T cells: Mechanisms and T-cell targets of suppression. Dev. Comp. Immunol. 2011, 35, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Boer, M.C.; Joosten, S.A.; Ottenhoff, T.H. Regulatory T-Cells at the Interface between Human Host and Pathogens in Infectious Diseases and Vaccination. Front. Immunol. 2015, 6, 217. [Google Scholar] [CrossRef] [Green Version]
- Silva-Campa, E.; Flores-Mendoza, L.; Resendiz, M.; Pinelli-Saavedra, A.; Mata-Haro, V.; Mwangi, W.; Hernandez, J. Induction of T helper 3 regulatory cells by dendritic cells infected with porcine reproductive and respiratory syndrome virus. Virology 2009, 387, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Campa, E.; Mata-Haro, V.; Mateu, E.; Hernandez, J. Porcine reproductive and respiratory syndrome virus induces CD4+CD8+CD25+Foxp3+ regulatory T cells (Tregs). Virology 2012, 430, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Wongyanin, P.; Buranapraditkun, S.; Chokeshai-Usaha, K.; Thanawonguwech, R.; Suradhat, S. Induction of inducible CD4+CD25+Foxp3+ regulatory T lymphocytes by porcine reproductive and respiratory syndrome virus (PRRSV). Vet. Immunol. Immunopathol. 2010, 133, 170–182. [Google Scholar] [CrossRef]
- Nedumpun, T.; Sirisereewan, C.; Thanmuan, C.; Techapongtada, P.; Puntarotairung, R.; Naraprasertkul, S.; Thanawongnuwech, R.; Suradhat, S. Induction of porcine reproductive and respiratory syndrome virus (PRRSV)-specific regulatory T lymphocytes (Treg) in the lungs and tracheobronchial lymph nodes of PRRSV-infected pigs. Vet. Microbiol. 2018, 216, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Nedumpun, T.; Techakriengkrai, N.; Thanawongnuwech, R.; Suradhat, S. Negative Immunomodulatory Effects of Type 2 Porcine Reproductive and Respiratory Syndrome Virus-Induced Interleukin-1 Receptor Antagonist on Porcine Innate and Adaptive Immune Functions. Front. Immunol. 2019, 10, 579. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, I.M.; Käser, T.; Gomez-Laguna, J.; Lamp, B.; Sinn, L.; Rümenapf, T.; Carrasco, L.; Saalmüller, A.; Gerner, W. PRRSV-infected monocyte-derived dendritic cells express high levels of SLA-DR and CD80/86 but do not stimulate PRRSV-naive regulatory T cells to proliferate. Vet. Res. 2015, 46, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kick, A.R.; Amaral, A.F.; Cortes, L.M.; Fogle, J.E.; Crisci, E.; Almond, G.W.; Käser, T. The T-Cell Response to Type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). Viruses 2019, 11, 796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocard, L.V.; Kick, A.R.; Hug, C.; Lischer, H.E.L.; Käser, T.; Summerfield, A. Systems Immunology Analyses Following Porcine Respiratory and Reproductive Syndrome Virus Infection and Vaccination. Front. Immunol. 2021, 12, 779747. [Google Scholar] [CrossRef]
- Butler, J.E.; Sinkora, M. The isolator piglet: A model for studying the development of adaptive immunity. Immunol. Res. 2007, 39, 33–51. [Google Scholar] [CrossRef]
- Poonsuk, K.; Zimmerman, J. Historical and contemporary aspects of maternal immunity in swine. Anim. Health Res. Rev. 2018, 19, 31–45. [Google Scholar] [CrossRef]
- Houben, S.; van Reeth, K.; Pensaert, M.B. Pattern of infection with the porcine reproductive and respiratory syndrome virus on swine farms in Belgium. Zent. Vet. B 1995, 42, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Tsukahara, T.; Imaoka, T.; Nakanishi, N.; Ushida, K.; Inoue, R. The effect of colostrum ingestion during the first 24 hours of life on early postnatal development of piglet immune systems. Anim. Sci. J. 2016, 87, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, C.M.T.; Payne, B.J.; Seate, J.L.; Murtaugh, M.P. Effect of Maternal Antibody Transfer on Antibody Dynamics and Control of Porcine Circovirus Type 2 Infection in Offspring. Viral Immunol. 2018, 31, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Nechvatalova, K.; Kudlackova, H.; Leva, L.; Babickova, K.; Faldyna, M. Transfer of humoral and cell-mediated immunity via colostrum in pigs. Vet. Immunol. Immunopathol. 2011, 142, 95–100. [Google Scholar] [CrossRef]
- Martin-Valls, G.E.; Mortensen, P.; Clilvert, H.; Li, Y.; Cortey, M.; Sno, M.; Barna, T.; Terre, M.; Guerra, N.; Mateu, E. The use of a whole inactivated PRRS virus vaccine administered in sows and impact on maternally derived immunity and timing of PRRS virus infection in piglets. Vet. Rec. Open 2022, 9, e34. [Google Scholar] [CrossRef]
- Kick, A.R.; Wolfe, Z.C.; Amaral, A.F.; Cortes, L.M.; Almond, G.W.; Crisci, E.; Gauger, P.C.; Pittman, J.; Käser, T. Maternal Autogenous Inactivated Virus Vaccination Boosts Immunity to PRRSV in Piglets. Vaccines 2021, 9, 106. [Google Scholar] [CrossRef]
- Bandrick, M.; Pieters, M.; Pijoan, C.; Molitor, T.W. Passive transfer of maternal Mycoplasma hyopneumoniae-specific cellular immunity to piglets. Clin. Vaccine Immunol. 2008, 15, 540–543. [Google Scholar] [CrossRef] [Green Version]
- Bandrick, M.; Ariza-Nieto, C.; Baidoo, S.K.; Molitor, T.W. Colostral antibody-mediated and cell-mediated immunity contributes to innate and antigen-specific immunity in piglets. Dev. Comp. Immunol. 2014, 43, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geldhof, M.F.; Van Breedam, W.; De Jong, E.; Lopez Rodriguez, A.; Karniychuk, U.U.; Vanhee, M.; Van Doorsselaere, J.; Maes, D.; Nauwynck, H.J. Antibody response and maternal immunity upon boosting PRRSV-immune sows with experimental farm-specific and commercial PRRSV vaccines. Vet. Microbiol. 2013, 167, 260–271. [Google Scholar] [CrossRef]
- Yang, S.; Kang, I.; Cho, H.; Oh, T.; Park, K.H.; Min, K.D.; Chae, C. A modified-live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine protects late-term pregnancy gilts against a heterologous PRRSV-2 challenge. Can. J Vet. Res. 2020, 84, 172–180. [Google Scholar]
- Bähr, A.; Käser, T.; Kemter, E.; Gerner, W.; Kurome, M.; Baars, W.; Herbach, N.; Witter, K.; Wünsch, A.; Talker, S.C.; et al. Ubiquitous LEA29Y Expression Blocks T Cell Co-Stimulation but Permits Sexual Reproduction in Genetically Modified Pigs. PLoS ONE 2016, 11, e0155676. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.; Kim, S.; Park, K.H.; Kang, I.; Park, S.J.; Yang, S.; Oh, T.; Chae, C. Vaccination with a porcine reproductive and respiratory syndrome virus vaccine at 1-day-old improved growth performance of piglets under field conditions. Vet. Microbiol. 2018, 214, 113–124. [Google Scholar] [CrossRef]
- Balasch, M.; Fort, M.; Taylor, L.P.; Diaz, I.; Mateu, E.; Calvert, J.G. Immune response development after vaccination of 1-day-old naive pigs with a Porcine Reproductive and Respiratory Syndrome 1-based modified live virus vaccine. Porcine Health Manag. 2019, 5, 2. [Google Scholar] [CrossRef]
- Balasch, M.; Fort, M.; Taylor, L.P.; Calvert, J.G. Vaccination of 1-day-old pigs with a porcine reproductive and respiratory syndrome virus (PRRSV) modified live attenuated virus vaccine is able to overcome maternal immunity. Porcine Health Manag. 2018, 4, 25. [Google Scholar] [CrossRef]
- Kreutzmann, H.; Durlinger, S.; Knecht, C.; Koch, M.; Cabana, M.; Torrent, G.; Balasch, M.; Taylor, L.P.; Balka, G.; Gerner, W.; et al. Efficacy of a Modified Live Virus Vaccine against Porcine Reproductive and Respiratory Syndrome Virus 1 (PRRSV-1) Administered to 1-Day-Old Piglets in Front of Heterologous PRRSV-1 Challenge. Pathogens 2021, 10, 1342. [Google Scholar] [CrossRef]
- Talker, S.C.; Käser, T.; Reutner, K.; Sedlak, C.; Mair, K.H.; Koinig, H.; Graage, R.; Viehmann, M.; Klingler, E.; Ladinig, A.; et al. Phenotypic maturation of porcine NK- and T-cell subsets. Dev. Comp. Immunol. 2013, 40, 51–68. [Google Scholar] [CrossRef]
- Gerner, W.; Talker, S.C.; Koinig, H.C.; Sedlak, C.; Mair, K.H.; Saalmüller, A. Phenotypic and functional differentiation of porcine alphabeta T cells: Current knowledge and available tools. Mol. Immunol. 2015, 66, 3–13. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Y.; Tong, G. Mechanisms of suppression of interferon production by porcine reproductive and respiratory syndrome virus. Acta Virol. 2012, 56, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Han, M.; Kim, C.; Calvert, J.G.; Yoo, D. Interplay between interferon-mediated innate immunity and porcine reproductive and respiratory syndrome virus. Viruses 2012, 4, 424–446. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Lopez, O.J.; Osorio, F.A. Role of neutralizing antibodies in PRRSV protective immunity. Vet. Immunol. Immunopathol. 2004, 102, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Wu, C.; Gu, G.; Sun, W.; Zhang, Y.J.; Zhou, E.M. Improved Vaccine against PRRSV: Current Progress and Future Perspective. Front. Microbiol. 2017, 8, 1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, I.J.; Joo, H.S.; Goyal, S.M.; Molitor, T.W. A modified serum neutralization test for the detection of antibody to porcine reproductive and respiratory syndrome virus in swine sera. J. Vet. Diagn. Investig. 1994, 6, 289–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, K.J.; Zimmerman, J.J.; Swenson, S.L.; McGinley, M.J.; Eernisse, K.A.; Brevik, A.; Rhinehart, L.L.; Frey, M.L.; Hill, H.T.; Platt, K.B. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J. Vet. Diagn. Investig. 1995, 7, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Labarque, G.G.; Nauwynck, H.J.; Van Reeth, K.; Pensaert, M.B. Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. J. Gen. Virol. 2000, 81, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.J.; Wu, L.L.; Zimmerman, J.J.; Hill, H.T.; Platt, K.B. Antibody-dependent enhancement (ADE) of porcine reproductive and respiratory syndrome virus (PRRSV) infection in pigs. Viral Immunol. 1996, 9, 51–63. [Google Scholar] [CrossRef]
- Osorio, F.A.; Galeota, J.A.; Nelson, E.; Brodersen, B.; Doster, A.; Wills, R.; Zuckermann, F.; Laegreid, W.W. Passive transfer of virus-specific antibodies confers protection against reproductive failure induced by a virulent strain of porcine reproductive and respiratory syndrome virus and establishes sterilizing immunity. Virology 2002, 302, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Lopez, O.J.; Oliveira, M.F.; Garcia, E.A.; Kwon, B.J.; Doster, A.; Osorio, F.A. Protection against porcine reproductive and respiratory syndrome virus (PRRSV) infection through passive transfer of PRRSV-neutralizing antibodies is dose dependent. Clin. Vaccine Immunol. 2007, 14, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.R.; Li, J.; Nelson, E.A.; Murtaugh, M.P. Broadly neutralizing antibodies against the rapidly evolving porcine reproductive and respiratory syndrome virus. Virus Res. 2015, 203, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.R.; Rahe, M.C.; Gray, D.K.; Martins, K.V.; Murtaugh, M.P. Porcine reproductive and respiratory syndrome virus neutralizing antibodies provide in vivo cross-protection to PRRSV1 and PRRSV2 viral challenge. Virus Res. 2018, 248, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Rahe, M.C.; Murtaugh, M.P. Interleukin-21 Drives Proliferation and Differentiation of Porcine Memory B Cells into Antibody Secreting Cells. PLoS ONE 2017, 12, e0171171. [Google Scholar] [CrossRef] [Green Version]
- Rahe, M.C.; Gustafson, K.L.; Murtaugh, M.P. B Cell Tetramer Development for Veterinary Vaccinology. Viral Immunol. 2018, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Zhao, X.; Yuan, M.; Zhang, K.; Dai, J.; Guan, X.; Qiu, H.-J.; Li, Y. Antibody-Dependent Enhancement: “Evil” Antibodies Favorable for Viral Infections. Viruses 2022, 14, 1739. [Google Scholar] [CrossRef]
- Cancel-Tirado, S.M.; Evans, R.B.; Yoon, K.J. Monoclonal antibody analysis of porcine reproductive and respiratory syndrome virus epitopes associated with antibody-dependent enhancement and neutralization of virus infection. Vet. Immunol. Immunopathol. 2004, 102, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Li, W.; Feng, X.; Han, F.; Zhang, Y.; Chen, J.; Liu, D.; Xia, P. Activating Fc Gamma Receptors and Viral Receptors Are Required for Antibody-Dependent Enhancement of Porcine Reproductive and Respiratory Syndrome Virus Infection. Vet. Sci. 2022, 9, 470. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, J.; Wang, D.; Li, N.; Qin, Y.; Du, D.; Yang, M.; Xia, P. Ligation of porcine Fc gamma receptor III inhibits levels of antiviral cytokine in response to PRRSV infection in vitro. Res. Vet. Sci. 2016, 105, 47–52. [Google Scholar] [CrossRef]
- Yang, S.; Kang, I.; Jeong, J.; Oh, T.; Park, K.H.; Park, S.J.; Ham, H.J.; Jin, G.R.; Lee, B.H.; Chae, C. A comparison of two commercially available porcine reproductive and respiratory syndrome virus (PRRSV) modified-live virus vaccines analyzing the growth performance in 1-day-old vaccinated swine located on endemic farms co-circulating PRRSV-1 and PRRSV-2. J. Vet. Med. Sci. 2019, 82, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Madapong, A.; Saeng-Chuto, K.; Chaikhumwang, P.; Tantituvanont, A.; Saardrak, K.; Pedrazuela Sanz, R.; Miranda Alvarez, J.; Nilubol, D. Immune response and protective efficacy of intramuscular and intradermal vaccination with porcine reproductive and respiratory syndrome virus 1 (PRRSV-1) modified live vaccine against highly pathogenic PRRSV-2 (HP-PRRSV-2) challenge, either alone or in combination with of PRRSV-1. Vet. Microbiol. 2020, 244, 108655. [Google Scholar] [CrossRef] [PubMed]
- Toman, M.; Celer, V.; Kavanová, L.; Levá, L.; Frolichova, J.; Ondráčková, P.; Kudláčková, H.; Nechvátalová, K.; Salat, J.; Faldyna, M. Dynamics and Differences in Systemic and Local Immune Responses After Vaccination with Inactivated and Live Commercial Vaccines and Subsequent Subclinical Infection with PRRS Virus. Front. Immunol. 2019, 10, 1689. [Google Scholar] [CrossRef] [Green Version]
- Sirisereewan, C.; Nedumpun, T.; Kesdangsakonwut, S.; Woonwong, Y.; Kedkovid, R.; Arunorat, J.; Thanawongnuwech, R.; Suradhat, S. Positive immunomodulatory effects of heterologous DNA vaccine- modified live vaccine, prime-boost immunization, against the highly-pathogenic PRRSV infection. Vet. Immunol. Immunopathol. 2017, 183, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Choi, K.; Kang, I.; Park, C.; Chae, C. Evaluation of a 20year old porcine reproductive and respiratory syndrome (PRRS) modified live vaccine (Ingelvac((R)) PRRS MLV) against two recent type 2 PRRS virus isolates in South Korea. Vet. Microbiol. 2016, 192, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, Y.; Zhang, C.; Tu, Y.; Tong, J.; Liu, Y.; Chang, Y.; Jiang, C.; Wang, S.; Zhou, E.M.; et al. Immune responses to modified live virus vaccines developed from classical or highly pathogenic PRRSV following challenge with a highly pathogenic PRRSV strain. Dev. Comp. Immunol. 2016, 62, 1–7. [Google Scholar] [CrossRef]
- Park, C.; Baek, J.H.; Cho, S.H.; Jeong, J.; Chae, C.; You, S.H.; Cha, S.H. Field porcine reproductive and respiratory syndrome viruses (PRRSV) attenuated by codon pair deoptimization (CPD) in NSP1 protected pigs from heterologous challenge. Virology 2020, 540, 172–183. [Google Scholar] [CrossRef]
- Evenson, D.; Gerber, P.F.; Xiao, C.T.; Halbur, P.G.; Wang, C.; Tian, D.; Ni, Y.Y.; Meng, X.J.; Opriessnig, T. A porcine reproductive and respiratory syndrome virus candidate vaccine based on the synthetic attenuated virus engineering approach is attenuated and effective in protecting against homologous virus challenge. Vaccine 2016, 34, 5546–5553. [Google Scholar] [CrossRef]
- Ni, Y.Y.; Zhao, Z.; Opriessnig, T.; Subramaniam, S.; Zhou, L.; Cao, D.; Cao, Q.; Yang, H.; Meng, X.J. Computer-aided codon-pairs deoptimization of the major envelope GP5 gene attenuates porcine reproductive and respiratory syndrome virus. Virology 2014, 450-451, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvert, J.G.; Keith, M.L.; Pearce, D.S.; Lenz, M.C.; King, V.L.; Diamondidis, Y.A.; Ankenbauer, R.G.; Martinon, N.C. Vaccination against porcine reproductive and respiratory syndrome virus (PRRSV) reduces the magnitude and duration of viremia following challenge with a virulent heterologous field strain. Vet. Microbiol. 2017, 205, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lobo, F.J.; Diez-Fuertes, F.; Simarro, I.; Castro, J.M.; Prieto, C. The Ability of Porcine Reproductive and Respiratory Syndrome Virus Isolates to Induce Broadly Reactive Neutralizing Antibodies Correlates with in Vivo Protection. Front. Immunol. 2021, 12, 691145. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Oh, T.; Cho, H.; Chae, C. A comparison of commercial modified-live PRRSV-1 and PRRSV-2 vaccines against a dual heterologous PRRSV-1 and PRRSV-2 challenge in late term pregnancy gilts. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101423. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, S.; Park, K.H.; Kang, I.; Park, S.J.; Park, C.; Chae, C. Evaluation of the effect of a porcine reproductive and respiratory syndrome (PRRS) modified-live virus vaccine on sow reproductive performance in endemic PRRS farms. Vet. Microbiol. 2017, 208, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Chaikhumwang, P.; Madapong, A.; Saeng-Chuto, K.; Nilubol, D.; Tantituvanont, A. Intranasal delivery of inactivated PRRSV loaded cationic nanoparticles coupled with enterotoxin subunit B induces PRRSV-specific immune responses in pigs. Sci. Rep. 2022, 12, 3725. [Google Scholar] [CrossRef]
- Yuan, F.; Sharma, J.; Nanjappa, S.G.; Gaulke, C.A.; Fang, Y. Effect of Killed PRRSV Vaccine on Gut Microbiota Diversity in Pigs. Viruses 2022, 14, 1081. [Google Scholar] [CrossRef]
- Correas, I.; Osorio, F.A.; Steffen, D.; Pattnaik, A.K.; Vu, H.L. Cross reactivity of immune responses to porcine reproductive and respiratory syndrome virus infection. Vaccine 2017, 35, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Fontanella, E.; Ma, Z.; Zhang, Y.; de Castro, A.M.; Shen, H.; Halbur, P.G.; Opriessnig, T. An interferon inducing porcine reproductive and respiratory syndrome virus vaccine candidate elicits protection against challenge with the heterologous virulent type 2 strain VR-2385 in pigs. Vaccine 2017, 35, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Cao, D.; Lynn Heffron, C.; Yugo, D.M.; Rogers, A.J.; Overend, C.; Matzinger, S.R.; Subramaniam, S.; Opriessnig, T.; LeRoith, T.; et al. Enhancing heterologous protection in pigs vaccinated with chimeric porcine reproductive and respiratory syndrome virus containing the full-length sequences of shuffled structural genes of multiple heterologous strains. Vaccine 2017, 35, 2427–2434. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Khatun, A.; Kim, W.I.; Cooper, V.; Cho, Y.I.; Wang, C.; Choi, E.J.; Yoon, K.J. Attempts to enhance cross-protection against porcine reproductive and respiratory syndrome viruses using chimeric viruses containing structural genes from two antigenically distinct strains. Vaccine 2016, 34, 4335–4342. [Google Scholar] [CrossRef]
- Oh, T.; Kim, H.; Park, K.H.; Jeong, J.; Kang, I.; Yang, S.; Chae, C. Effectiveness of a commercial porcine reproductive and respiratory syndrome virus (PRRSV) subunit vaccine against heterologous PRRSV-1 and PRRSV-2 challenge in late-term pregnant gilts. Can. J. Vet. Res. 2019, 83, 248–254. [Google Scholar]
- Martinez-Lobo, F.J.; Diez-Fuertes, F.; Simarro, I.; Castro, J.M.; Prieto, C. Porcine Reproductive and Respiratory Syndrome Virus isolates differ in their susceptibility to neutralization. Vaccine 2011, 29, 6928–6940. [Google Scholar] [CrossRef]
- Mötz, M.; Stas, M.; Hammer, S.; Duckova, T.; Fontaine, F.; Kiesler, A.; Seitz, K.; Ladinig, A.; Müller, A.; Riedel, C.; et al. Identification of MHC-I-Presented Porcine Respiratory and Reproductive Syndrome Virus (PRRSV) Peptides Reveals Immunogenic Epitopes within Several Non-Structural Proteins Recognized by CD8+ T Cells. Viruses 2022, 14, 1891. [Google Scholar] [CrossRef]
- Proctor, J.; Wolf, I.; Brodsky, D.; Cortes, L.M.; Frias-De-Diego, A.; Almond, G.W.; Crisci, E.; Negrao Watanabe, T.T.; Hammer, J.M.; Käser, T. Heterologous vaccine immunogenicity, efficacy, and immune correlates of protection of a modified-live virus porcine reproductive and respiratory syndrome virus vaccine. Front. Microbiol. 2022, 13, 977796. [Google Scholar] [CrossRef] [PubMed]
- Kick, A.R.; Amaral, A.F.; Frias-De-Diego, A.; Cortes, L.M.; Fogle, J.E.; Crisci, E.; Almond, G.W.; Käser, T. The Local and Systemic Humoral Immune Response Against Homologous and Heterologous Strains of the Type 2 Porcine Reproductive and Respiratory Syndrome Virus. Front. Immunol. 2021, 12, 637613. [Google Scholar] [CrossRef] [PubMed]
- Bernelin-Cottet, C.; Urien, C.; Stubsrud, E.; Jakob, V.; Bouguyon, E.; Bordet, E.; Barc, C.; Boulesteix, O.; Contreras, V.; Barnier-Quer, C.; et al. A DNA-Modified Live Vaccine Prime-Boost Strategy Broadens the T-Cell Response and Enhances the Antibody Response against the Porcine Reproductive and Respiratory Syndrome Virus. Viruses 2019, 11, 551. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, J.; Ferlazzo, G.; Boniotti, M.B.; Capucci, L.; Guarneri, F.; Barbieri, I.; Alborali, G.L.; Amadori, M. Characterization of the IgA response to PRRS virus in pig oral fluids. PLoS ONE 2020, 15, e0229065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrarini, G.; Borghetti, P.; De Angelis, E.; Ferrari, L.; Canelli, E.; Catella, A.; Di Lecce, R.; Martelli, P. Immunoregulatory signal FoxP3, cytokine gene expression and IFN-gamma cell responsiveness upon porcine reproductive and respiratory syndrome virus (PRRSV) natural infection. Res. Vet. Sci. 2015, 103, 96–102. [Google Scholar] [CrossRef]
- Hermann, J.R.; Munoz-Zanzi, C.A.; Roof, M.B.; Burkhart, K.; Zimmerman, J.J. Probability of porcine reproductive and respiratory syndrome (PRRS) virus infection as a function of exposure route and dose. Vet. Microbiol. 2005, 110, 7–16. [Google Scholar] [CrossRef]
- Eclercy, J.; Renson, P.; Lebret, A.; Hirchaud, E.; Normand, V.; Andraud, M.; Paboeuf, F.; Blanchard, Y.; Rose, N.; Bourry, O. A Field Recombinant Strain Derived from Two Type 1 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV-1) Modified Live Vaccines Shows Increased Viremia and Transmission in SPF Pigs. Viruses 2019, 11, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renson, P.; Fablet, C.; Andraud, M.; Normand, V.; Lebret, A.; Paboeuf, F.; Rose, N.; Bourry, O. Maternally-derived neutralizing antibodies reduce vaccine efficacy against porcine reproductive and respiratory syndrome virus infection. Vaccine 2019, 37, 4318–4324. [Google Scholar] [CrossRef] [PubMed]
- Nodelijk, G.; de Jong, M.C.; van Leengoed, L.A.; Wensvoort, G.; Pol, J.M.; Steverink, P.J.; Verheijden, J.H. A quantitative assessment of the effectiveness of PRRSV vaccination in pigs under experimental conditions. Vaccine 2001, 19, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Vaccines: Correlates of vaccine-induced immunity. Clin. Infect. Dis. 2008, 47, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Alex Pasternak, J.; MacPhee, D.J.; Harding, J.C.S. Fetal cytokine response to porcine reproductive and respiratory syndrome virus-2 infection. Cytokine 2020, 126, 154883. [Google Scholar] [CrossRef]
- Renson, P.; Rose, N.; Le Dimna, M.; Mahe, S.; Keranflec’h, A.; Paboeuf, F.; Belloc, C.; Le Potier, M.F.; Bourry, O. Dynamic changes in bronchoalveolar macrophages and cytokines during infection of pigs with a highly or low pathogenic genotype 1 PRRSV strain. Vet. Res. 2017, 48, 15. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, V.; Manickam, C.; Binjawadagi, B.; Linhares, D.; Murtaugh, M.P.; Renukaradhya, G.J. Evaluation of immune responses to porcine reproductive and respiratory syndrome virus in pigs during early stage of infection under farm conditions. Virol. J. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, I.; Gimeno, M.; Darwich, L.; Navarro, N.; Kuzemtseva, L.; Lopez, S.; Galindo, I.; Segales, J.; Martin, M.; Pujols, J.; et al. Characterization of homologous and heterologous adaptive immune responses in porcine reproductive and respiratory syndrome virus infection. Vet. Res. 2012, 43, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Käser, T.; Mair, K.H.; Hammer, S.E.; Gerner, W.; Saalmüller, A. Natural and inducible Tregs in swine: Helios expression and functional properties. Dev. Comp. Immunol. 2015, 49, 323–331. [Google Scholar] [CrossRef]
- Käser, T.; Gerner, W.; Mair, K.; Hammer, S.E.; Patzl, M.; Saalmüller, A. Current knowledge on porcine regulatory T cells. Vet. Immunol. Immunopathol. 2012, 148, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Diaz, I.; Darwich, L.; Pappaterra, G.; Pujols, J.; Mateu, E. Immune responses of pigs after experimental infection with a European strain of Porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2005, 86, 1943–1951. [Google Scholar] [CrossRef]
- Albina, E.; Piriou, L.; Hutet, E.; Cariolet, R.; L’Hospitalier, R. Immune responses in pigs infected with porcine reproductive and respiratory syndrome virus (PRRSV). Vet. Immunol. Immunopathol. 1998, 61, 49–66. [Google Scholar] [CrossRef]
- Ladinig, A.; Gerner, W.; Saalmüller, A.; Lunney, J.K.; Ashley, C.; Harding, J.C. Changes in leukocyte subsets of pregnant gilts experimentally infected with porcine reproductive and respiratory syndrome virus and relationships with viral load and fetal outcome. Vet. Res. 2014, 45, 128. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, V.; Manickam, C.; Patterson, R.; Dodson, K.; Murtaugh, M.; Torrelles, J.B.; Schlesinger, L.S.; Renukaradhya, G.J. Cross-protective immunity to porcine reproductive and respiratory syndrome virus by intranasal delivery of a live virus vaccine with a potent adjuvant. Vaccine 2011, 29, 4058–4066. [Google Scholar] [CrossRef]
- Ferrari, L.; Canelli, E.; De Angelis, E.; Catella, A.; Ferrarini, G.; Ogno, G.; Bonati, L.; Nardini, R.; Borghetti, P.; Martelli, P. A highly pathogenic porcine reproductive and respiratory syndrome virus type 1 (PRRSV-1) strongly modulates cellular innate and adaptive immune subsets upon experimental infection. Vet. Microbiol. 2018, 216, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Nazki, S.; Khatun, A.; Jeong, C.G.; Mattoo, S.U.S.; Gu, S.; Lee, S.I.; Kim, S.C.; Park, J.H.; Yang, M.S.; Kim, B.; et al. Evaluation of local and systemic immune responses in pigs experimentally challenged with porcine reproductive and respiratory syndrome virus. Vet. Res. 2020, 51, 66. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, L.; Page, C.; Larochelle, R.; Magar, R. Porcine reproductive and respiratory syndrome virus persistence in blood, spleen, lymph nodes, and tonsils of experimentally infected pigs depends on the level of CD8high T cells. Viral Immunol. 2003, 16, 395–406. [Google Scholar] [CrossRef]
- Bautista, E.M.; Molitor, T.W. Cell-mediated immunity to porcine reproductive and respiratory syndrome virus in swine. Viral Immunol. 1997, 10, 83–94. [Google Scholar] [CrossRef]
- Meier, W.A.; Galeota, J.; Osorio, F.A.; Husmann, R.J.; Schnitzlein, W.M.; Zuckermann, F.A. Gradual development of the interferon-gamma response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology 2003, 309, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Costers, S.; Lefebvre, D.J.; Goddeeris, B.; Delputte, P.L.; Nauwynck, H.J. Functional impairment of PRRSV-specific peripheral CD3+CD8high cells. Vet. Res. 2009, 40, 46. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, L.; Martelli, P.; Saleri, R.; De Angelis, E.; Cavalli, V.; Bresaola, M.; Benetti, M.; Borghetti, P. Lymphocyte activation as cytokine gene expression and secretion is related to the porcine reproductive and respiratory syndrome virus (PRRSV) isolate after in vitro homologous and heterologous recall of peripheral blood mononuclear cells (PBMC) from pigs vaccinated and exposed to natural infection. Vet. Immunol. Immunopathol. 2013, 151, 193–206. [Google Scholar] [CrossRef]
- Park, C.; Seo, H.W.; Han, K.; Kang, I.; Chae, C. Evaluation of the efficacy of a new modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine (Fostera PRRS) against heterologous PRRSV challenge. Vet. Microbiol. 2014, 172, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Mair, K.H.; Koinig, H.; Gerner, W.; Hohne, A.; Bretthauer, J.; Kroll, J.J.; Roof, M.B.; Saalmüller, A.; Stadler, K.; Libanova, R. Carbopol improves the early cellular immune responses induced by the modified-life vaccine Ingelvac PRRS(R) MLV. Vet. Microbiol. 2015, 176, 352–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madapong, A.; Saeng-Chuto, K.; Boonsoongnern, A.; Tantituvanont, A.; Nilubol, D. Cell-mediated immune response and protective efficacy of porcine reproductive and respiratory syndrome virus modified-live vaccines against co-challenge with PRRSV-1 and PRRSV-2. Sci. Rep. 2020, 10, 1649. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; O’Connell, C.M.; Hagen, C.; Sawicki, K.; Smyth, J.A.; Verardi, P.H.; Kruiningen, H.J.V.; Garmendia, A.E. Broad Protection of Pigs against Heterologous PRRSV Strains by a GP5-Mosaic DNA Vaccine Prime/GP5-Mosaic rVaccinia (VACV) Vaccine Boost. Vaccines 2020, 8, 106. [Google Scholar] [CrossRef] [Green Version]
- Vreman, S.; Stockhofe-Zurwieden, N.; Popma-de Graaf, D.J.; Savelkoul, H.F.J.; Barnier-Quer, C.; Collin, N.; Collins, D.; McDaid, D.; Moore, A.C.; Rebel, J.M.J. Immune responses induced by inactivated porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in neonatal pigs using different adjuvants. Vet. Immunol. Immunopathol. 2021, 232, 110170. [Google Scholar] [CrossRef]
- Chrun, T.; Maze, E.A.; Vatzia, E.; Martini, V.; Paudyal, B.; Edmans, M.D.; McNee, A.; Manjegowda, T.; Salguero, F.J.; Wanasen, N.; et al. Simultaneous Infection with Porcine Reproductive and Respiratory Syndrome and Influenza Viruses Abrogates Clinical Protection Induced by Live Attenuated Porcine Reproductive and Respiratory Syndrome Vaccination. Front. Immunol. 2021, 12, 758368. [Google Scholar] [CrossRef] [PubMed]
- Reutner, K.; Leitner, J.; Mullebner, A.; Ladinig, A.; Essler, S.E.; Duvigneau, J.C.; Ritzmann, M.; Steinberger, P.; Saalmüller, A.; Gerner, W. CD27 expression discriminates porcine T helper cells with functionally distinct properties. Vet. Res. 2013, 44, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Gomez, I.M.; Talker, S.C.; Käser, T.; Stadler, M.; Hammer, S.E.; Saalmüller, A.; Gerner, W. Expression of T-bet, Eomesodermin and GATA-3 in porcine alphabeta T cells. Dev. Comp. Immunol. 2016, 60, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gomez, I.M.; Talker, S.C.; Käser, T.; Stadler, M.; Reiter, L.; Ladinig, A.; Milburn, J.V.; Hammer, S.E.; Mair, K.H.; Saalmüller, A.; et al. Expression of T-Bet, Eomesodermin, and GATA-3 Correlates with Distinct Phenotypes and Functional Properties in Porcine gammadelta T Cells. Front. Immunol. 2019, 10, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.; Seo, H.W.; Park, C.; Chae, C. Vaccination of sows against type 2 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) before artificial insemination protects against type 2 PRRSV challenge but does not protect against type 1 PRRSV challenge in late gestation. Vet. Res. 2014, 45, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Galliher-Beckley, A.; Pappan, L.; Trible, B.; Kerrigan, M.; Beck, A.; Hesse, R.; Blecha, F.; Nietfeld, J.C.; Rowland, R.R.; et al. Comparison of host immune responses to homologous and heterologous type II porcine reproductive and respiratory syndrome virus (PRRSV) challenge in vaccinated and unvaccinated pigs. Biomed. Res. Int. 2014, 2014, 416727. [Google Scholar] [CrossRef] [Green Version]
- Molina, R.M.; Cha, S.H.; Chittick, W.; Lawson, S.; Murtaugh, M.P.; Nelson, E.A.; Christopher-Hennings, J.; Yoon, K.J.; Evans, R.; Rowland, R.R.; et al. Immune response against porcine reproductive and respiratory syndrome virus during acute and chronic infection. Vet. Immunol. Immunopathol. 2008, 126, 283–292. [Google Scholar] [CrossRef]
- Meier, W.A.; Husmann, R.J.; Schnitzlein, W.M.; Osorio, F.A.; Lunney, J.K.; Zuckermann, F.A. Cytokines and synthetic double-stranded RNA augment the T helper 1 immune response of swine to porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2004, 102, 299–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Oh, T.; Kang, I.; Park, S.J.; Chae, C. Efficacy of concurrent vaccination with modified-live PRRSV-1 and PRRSV-2 vaccines against heterologous dual PRRSV-1 and PRRSV-2 challenge in late term pregnancy gilts. Vet. Microbiol. 2019, 239, 108497. [Google Scholar] [CrossRef] [PubMed]
- Drigo, M.; Giacomini, E.; Lazzaro, M.; Pasotto, D.; Bilato, D.; Ruggeri, J.; Boniotti, M.B.; Alborali, G.L.; Amadori, M. Comparative evaluation of immune responses of swine in PRRS-stable and unstable herds. Vet. Immunol. Immunopathol. 2018, 200, 32–39. [Google Scholar] [CrossRef]
- Choi, K.; Park, C.; Jeong, J.; Chae, C. Comparison of protection provided by type 1 and type 2 porcine reproductive and respiratory syndrome field viruses against homologous and heterologous challenge. Vet. Microbiol. 2016, 191, 72–81. [Google Scholar] [CrossRef]
- Amaral, A.F.; Rahman, K.S.; Kick, A.R.; Cortes, L.M.; Robertson, J.; Kaltenboeck, B.; Gerdts, V.; O’Connell, C.M.; Poston, T.B.; Zheng, X.; et al. Mucosal Vaccination with UV-Inactivated Chlamydia suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-γ+ Effector Memory Cells. Vaccines 2020, 8, 353. [Google Scholar] [CrossRef]
- Zuckermann, F.A.; Garcia, E.A.; Luque, I.D.; Christopher-Hennings, J.; Doster, A.; Brito, M.; Osorio, F. Assessment of the efficacy of commercial porcine reproductive and respiratory syndrome virus (PRRSV) vaccines based on measurement of serologic response, frequency of gamma-IFN-producing cells and virological parameters of protection upon challenge. Vet. Microbiol. 2007, 123, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Renu, S.; Ghimire, S.; Shaan Lakshmanappa, Y.; Hogshead, B.T.; Feliciano-Ruiz, N.; Lu, F.; HogenEsch, H.; Krakowka, S.; Lee, C.W.; et al. Mucosal Immunity and Protective Efficacy of Intranasal Inactivated Influenza Vaccine Is Improved by Chitosan Nanoparticle Delivery in Pigs. Front. Immunol. 2018, 9, 934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charerntantanakul, W. Porcine reproductive and respiratory syndrome virus vaccines: Immunogenicity, efficacy and safety aspects. World J. Virol. 2012, 1, 23–30. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Meng, X.J.; Calvert, J.G.; Roof, M.; Lager, K.M. Live porcine reproductive and respiratory syndrome virus vaccines: Current status and future direction. Vaccine 2015, 33, 4069–4080. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Dwivedi, V.; Manickam, C.; Binjawadagi, B.; Benfield, D. Mucosal vaccines to prevent porcine reproductive and respiratory syndrome: A new perspective. Anim. Health Res. Rev. 2012, 13, 21–37. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, B.; Chang, H.; Zhang, R.; Lu, F.; Wang, F.; Fang, F.; Chen, Z. Intranasal Immunization of Mice to Avoid Interference of Maternal Antibody against H5N1 Infection. PLoS ONE 2016, 11, e0157041. [Google Scholar] [CrossRef] [Green Version]
- Madapong, A.; Saeng-Chuto, K.; Tantituvanont, A.; Nilubol, D. Using a concurrent challenge with porcine circovirus 2 and porcine reproductive and respiratory syndrome virus to compare swine vaccination programs. Sci. Rep. 2022, 12, 15524. [Google Scholar] [CrossRef]
- Renson, P.; Deblanc, C.; Bougon, J.; Le Dimna, M.; Gorin, S.; Mahe, S.; Barbier, N.; Paboeuf, F.; Simon, G.; Bourry, O. Concomitant Swine Influenza A Virus Infection Alters PRRSV1 MLV Viremia in Piglets but Does Not Interfere with Vaccine Protection in Experimental Conditions. Vaccines 2021, 9, 356. [Google Scholar] [CrossRef]
- Wu, C.; Gu, G.; Zhai, T.; Wang, Y.; Yang, Y.; Li, Y.; Zheng, X.; Zhao, Q.; Zhou, E.M.; Nan, Y. Broad neutralization activity against both PRRSV-1 and PRRSV-2 and enhancement of cell mediated immunity against PRRSV by a novel IgM monoclonal antibody. Antiviral Res 2020, 175, 104716. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Choi, K.; Jeong, J.; Chae, C. Cross-protection of a new type 2 porcine reproductive and respiratory syndrome virus (PRRSV) modified live vaccine (Fostera PRRS) against heterologous type 1 PRRSV challenge in growing pigs. Vet. Microbiol. 2015, 177, 87–94. [Google Scholar] [CrossRef]
- Martelli, P.; Ardigo, P.; Ferrari, L.; Morganti, M.; De Angelis, E.; Bonilauri, P.; Luppi, A.; Guazzetti, S.; Caleffi, A.; Borghetti, P. Concurrent vaccinations against PCV2 and PRRSV: Study on the specific immunity and clinical protection in naturally infected pigs. Vet. Microbiol. 2013, 162, 558–571. [Google Scholar] [CrossRef]
- Xiao, Z.; Batista, L.; Dee, S.; Halbur, P.; Murtaugh, M.P. The level of virus-specific T-cell and macrophage recruitment in porcine reproductive and respiratory syndrome virus infection in pigs is independent of virus load. J. Virol. 2004, 78, 5923–5933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos, R.G.; Dellagostin, O.A.; Barletta, R.G.; Doster, A.R.; Nelson, E.; Zuckermann, F.; Osorio, F.A. Immune response of pigs inoculated with Mycobacterium bovis BCG expressing a truncated form of GP5 and M protein of porcine reproductive and respiratory syndrome virus. Vaccine 2004, 22, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Meier, W.A.; Wheeler, J.; Husmann, R.J.; Osorio, F.A.; Zuckermann, F.A. Characteristics of the immune response of pigs to wild-type PRRS virus or to commercially available vaccines: An unconventional response. Proc. Am. Assoc. Swine Pract. 2000, 415–418. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kick, A.R.; Grete, A.F.; Crisci, E.; Almond, G.W.; Käser, T. Testable Candidate Immune Correlates of Protection for Porcine Reproductive and Respiratory Syndrome Virus Vaccination. Vaccines 2023, 11, 594. https://doi.org/10.3390/vaccines11030594
Kick AR, Grete AF, Crisci E, Almond GW, Käser T. Testable Candidate Immune Correlates of Protection for Porcine Reproductive and Respiratory Syndrome Virus Vaccination. Vaccines. 2023; 11(3):594. https://doi.org/10.3390/vaccines11030594
Chicago/Turabian StyleKick, Andrew R., Alicyn F. Grete, Elisa Crisci, Glen W. Almond, and Tobias Käser. 2023. "Testable Candidate Immune Correlates of Protection for Porcine Reproductive and Respiratory Syndrome Virus Vaccination" Vaccines 11, no. 3: 594. https://doi.org/10.3390/vaccines11030594