Analysing the In-Use Stability of mRNA-LNP COVID-19 Vaccines Comirnaty™ (Pfizer) and Spikevax™ (Moderna): A Comparative Study of the Particulate
Abstract
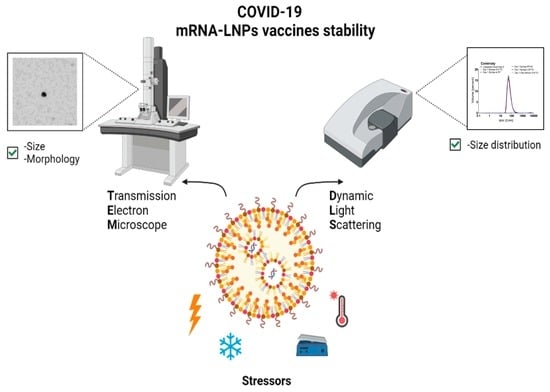
:1. Introduction
2. Materials and Methods
2.1. Vaccines, Solutions, and Materials
2.2. Stability Study
2.2.1. Aseptic Pharmaceutical Preparation
2.2.2. Stress Factors Study
2.2.3. Twenty-Four Hour Stability Study
2.3. Visual Characteristics
2.4. Dynamic Light Scattering (DLS)
2.5. Transmission Electron Microscopy (TEM)
2.6. Data Analysis
3. Results
3.1. Visual Characteristics
3.2. Dynamic Light Scattering
3.2.1. Characterisation of Comirnaty™ Clinical Dispersions: A Comparative Study of Unexpired and Expired Samples
3.2.2. Characterisation of Spikevax™ Clinical Dispersions: A Comparative Study of Unexpired and Expired Samples
3.2.3. Stress Factors Study
3.2.4. Comirnaty™ 24-h Stability Study (Unexpired Samples)
3.3. Transmission Electron Microscopy (TEM) Analysis
4. Discussion
5. Concluding Remarks
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Cantu, D.O.; Wang, X.; Carrasco-Magallanes, H.; Afewerki, S.; Zhang, X.; Bonventre, J.V.; Ruiz-Esparza, G.U. From Bench to the Clinic: The Path to Translation of Nanotechnology-Enabled MRNA SARS-CoV-2 Vaccines. Nanomicro Lett. 2022, 14, 41. [Google Scholar] [CrossRef]
- Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. Available online: https://www.who.int/en/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemicregulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic (accessed on 26 May 2023).
- From Emergency Response to Long-Term COVID-19 Disease Management: Sustaining Gains Made during the COVID-19 Pandemic. Available online: https://www.who.int/publications/i/item/WHO-WHE-SPP-2023.1 (accessed on 26 May 2023).
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Tracker|European Centre for Disease Prevention and Control. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#distribution-tab (accessed on 26 May 2023).
- Xu, Z.; Fisher, D.E. MRNA Melanoma Vaccine Revolution Spurred by the COVID-19 Pandemic. Front. Immunol. 2023, 14, 1155728. [Google Scholar] [CrossRef]
- Kloczewiak, M.; Banks, J.M.; Jin, L.; Brader, M.L. A Biopharmaceutical Perspective on Higher-Order Structure and Thermal Stability of MRNA Vaccines. Mol. Pharm. 2022, 19, 2022–2031. [Google Scholar] [CrossRef]
- Oude Blenke, E.; Örnskov, E.; Schöneich, C.; Nilsson, G.A.; Volkin, D.B.; Mastrobattista, E.; Almarsson, Ö.; Crommelin, D.J.A. The Storage and In-Use Stability of MRNA Vaccines and Therapeutics: Not A Cold Case. J. Pharm. Sci. 2023, 112, 386–403. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of MRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of MRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- European Medicines Agency. Annex I Summary of Product Characteristics: Comirnaty; European Medicines Agency: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Selmin, F.; Musazzi, U.M.; Franzè, S.; Scarpa, E.; Rizzello, L.; Procacci, P.; Minghetti, P. Pre-Drawn Syringes of Comirnaty for an Efficient COVID-19 Mass Vaccination: Demonstration of Stability. Pharmaceutics 2021, 13, 1029. [Google Scholar] [CrossRef]
- Kudsiova, L.; Lansley, A.; Scutt, G.; Allen, M.; Bowler, L.; Williams, S.; Lippett, S.; Stafford, S.; Tarzi, M.; Cross, M.; et al. Stability Testing of the Pfizer-BioNTech BNT162b2 COVID-19 Vaccine: A Translational Study in UK Vaccination Centres. BMJ Open Sci. 2021, 5, e100203. [Google Scholar] [CrossRef]
- Thaller, A.; Schmauder, L.; Frieß, W.; Winter, G.; Menzen, T.; Hawe, A.; Richter, K. SV-AUC as a Stability-Indicating Method for the Characterization of MRNA-LNPs. Eur. J. Pharm. Biopharm. 2023, 182, 152–156. [Google Scholar] [CrossRef]
- European Medicines Agency. Annex I Summary of Product Characteristics: Spikevax; European Medicines Agency: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Sato, Y.; Note, Y.; Maeki, M.; Kaji, N.; Baba, Y.; Tokeshi, M.; Harashima, H. Elucidation of the Physicochemical Properties and Potency of SiRNA-Loaded Small-Sized Lipid Nanoparticles for SiRNA Delivery. J. Control. Release 2016, 229, 48–57. [Google Scholar] [CrossRef]
- Chen, S.; Tam, Y.Y.C.; Lin, P.J.C.; Sung, M.M.H.; Tam, Y.K.; Cullis, P.R. Influence of Particle Size on the in Vivo Potency of Lipid Nanoparticle Formulations of SiRNA. J. Control. Release 2016, 235, 236–244. [Google Scholar] [CrossRef]
- Spikevax (Previously COVID-19 Vaccine Moderna)|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/spikevax#product-information-section (accessed on 26 May 2023).
- CHMP. Committee for Medicinal Products for Human Use (CHMP) Assessment Report Spikevax; European Medicines Agency: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of MRNA BNT162b2 COVID-19 Vaccine up to 6 Months in a Large Integrated Health System in the USA: A Retrospective Cohort Study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Grau, S.; Ferrández, O.; Martín-García, E.; Maldonado, R. Reconstituted MRNA COVID-19 Vaccines May Maintain Stability after Continuous Movement. Clin. Microbiol. Infect. 2021, 27, 1698.e1–1698.e4. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-Term Storage of Lipid-like Nanoparticles for MRNA Delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Ball, R.; Bajaj, P.; Whitehead, K. Achieving Long-Term Stability of Lipid Nanoparticles: Examining the Effect of PH, Temperature, and Lyophilization. Int. J. Nanomed. 2016, 12, 305–315. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A Comparison of TEM and DLS Methods to Characterize Size Distribution of Ceramic Nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef]
- Wilson, B.K.; Prud’homme, R.K. Nanoparticle Size Distribution Quantification from Transmission Electron Microscopy (TEM) of Ruthenium Tetroxide Stained Polymeric Nanoparticles. J. Colloid. Interface Sci. 2021, 604, 208–220. [Google Scholar] [CrossRef]
- Jankevics Jones, H.; Markova, N. Shining a Light on Lipid Nanoparticle Characterization. Pharm. Technol. 2022, 46, 20–23. [Google Scholar]
- Hassett, K.J.; Higgins, J.; Woods, A.; Levy, B.; Xia, Y.; Hsiao, C.J.; Acosta, E.; Almarsson, Ö.; Moore, M.J.; Brito, L.A. Impact of Lipid Nanoparticle Size on MRNA Vaccine Immunogenicity. J. Control. Release 2021, 335, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified MRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seth, A.; Wibowo, N.; Zhao, C.-X.; Mitter, N.; Yu, C.; Middelberg, A.P.J. Nanoparticle Vaccines. Vaccine 2014, 32, 327–337. [Google Scholar] [CrossRef] [PubMed]








| Vaccine | Expired Batches | Unexpired Batches |
|---|---|---|
| Comirnaty™ | FG4686 (Exp. 11/2021) 1 | FP8545 (Exp. 07/2022) 1 IK081A (Exp. 07/2022) 2 |
| Spikevax™ | 000095A (Exp. 05/2022) 1,3 | 3006323 (Exp. 05/2022) 1 |
| Parameters | Stress Test | Z-Average (nm) | PDI | Population 1 (Volume) | Population 2 (Volume) | Population 3 (Volume) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccines | Dh ± SD (nm) | vol ± SD (%) | Dh ± SD (nm) | vol ± SD (%) | Dh ± SD (nm) | vol ± SD (%) | ||||
| Pfizer-BioNTech (Comirnaty™) | Control 1 (E) | 147 ± 3 | 0.69 ± 0.04 | 64 ± 1 | 89.6 ± 0.7 | 700 ± 60 | 9 ± 1 | 4800 ± 600 | 2 ± 1 | |
| Artificial light 24 h | 146 ± 2 | 0.70 ± 0.04 | 63 ± 2 | 87.9 ± 0.3 | 600 ± 50 | 10.3 | 5000 ± 100 | 1.8 ± 0.3 | ||
| Control 2 (E) | 149 ± 4 | 0.70 ± 0.04 | 62 ± 2 | 88.60 ± 0.06 | 650 ± 40 | 9.7 ± 0.6 | 5100 ± 100 | 1.7 ± 0.6 | ||
| Manual shaking (E) | 170 ± 20 | 0.70 ± 0.10 | 61 ± 2 | 87.2 ± 0.3 | 680 ± 70 | 11.6 ± 0.9 | 5090 ± 30 | 1.3 ± 0.6 | ||
| Vortex vibration (E) | 165 ± 7 | 0.74 ± 0.04 | 63 ± 1 | 86 ± 1 | 400 ± 300 | 13 ± 1 * | 5100 ± 200 | 1.6 ± 0.3 | ||
| Control 3 (E) | 147.1 ± 0.6 | 0.71 ± 0.03 | 63 ± 2 | 88.9 ± 0.6 | 700 ± 10 | 9.3 ± 0.4 | 4990 ± 60 | 1.8 ± 0.6 | ||
| Syringe injection (1x) (E) | 149 ± 3 | 0.73 ± 0.01 | 62.7 ± 0.5 | 87.7 ± 0.7 | 620 ± 20 | 10.5 ± 0.8 | 5000 ± 100 | 1.7 ± 0.2 | ||
| Syringe injection (3x) (E) | 156 ± 6 | 0.66 ± 0.01 | 63 ± 2 | 86.9 ± 0.8 | 610 ± 30 | 12 ± 1 * | 5100 ± 40 | 1.5 ± 0.4 | ||
| Control 4 (NE) | 88 ± 1 | 0.23 ± 0.01 | 63.0 ± 0.9 | 99.40 ± 0.06 | NP | NP | 4400 ± 200 | 0.630 ± 0.06 | ||
| Manual shaking (NE) | 116 ± 8 * | 0.38 ± 0.02 * | 65.1 ± 0.5 | 94.4 ± 0.3 * | 600 ± 60 | 5.5 ± 0.3 | 4873 | 0.1 ± 0.2 | ||
| Vortex vibration (NE) | 96 ± 1 | 0.26 ± 0.01 | 65.2 ± 0.9 | 98.5 ± 0.6 | 1000 ± 200 | 1 ± 1 | 4571 | 0.3 ± 0.5 | ||
| Moderna (Spikevax™) | Control 1 (E) | 220 ± 2 | 0.22 ± 0.01 | |||||||
| Natural light 24 h (E) | 215 ± 2 | 0.22 ± 0.01 | ||||||||
| Control 2 (E) | 221 ± 4 | 0.21 ± 0.01 | ||||||||
| Manual shaking (E) | 222 ± 3 | 0.23 ± 0.01 | ||||||||
| Vortex vibration (E) | 220 ± 4 | 0.22 ± 0.01 | ||||||||
| Control 3 (E) | 220 ± 2 | 0.22 ± 0.01 | ||||||||
| Syringe injection (1x) (E) | 220 ± 2 | 0.22 ± 0.01 | ||||||||
| Syringe injection (3x) (E) | 219 ± 3 | 0.22 ± 0.01 | ||||||||
| Control 4 (NE) | 218 ± 1 | 0.220 ± 0.003 | ||||||||
| Manual shaking (NE) | 222 ± 3 | 0.23 ± 0.01 | ||||||||
| Vortex vibration (NE) | 216 ± 2 | 0.21 ± 0.01 | ||||||||
| Parameters | Storage Condition | Z-Average (nm) | PDI | Population 1 | Population 2 | Population 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine | Dh ± SD (nm) | vol ± SD (%) | Dh ± SD (nm) | vol ± SD (%) | Dh ± SD (nm) | vol ± SD (%) | ||||
| Pfizer-BioNTech (Comirnaty™) | Control (unexpired) | 89.3 ± 0.9 | 0.20 | 66 ± 1 | 99.7 ± 0.1 | NP | NP | 4900 ± 100 | 0.4 ± 0.1 | |
| Syringe 24 h 2–8 °C | 90 ± 1 | 0.20 | 65.7 ± 0.5 | 99.7 ± 0.1 | NP | NP | 4600 ± 400 | 0.3 ± 0.1 | ||
| Syringe 24 h RT/AC (20 °C) | 91 ± 1 | 0.22 | 67 ± 1 | 99.4 | NP | NP | 4810 ± 90 | 0.60 | ||
| Syringe 24 h RT | 91 ± 1 | 0.21 | 65.5 ± 0.7 | 99.4 ± 0.1 | NP | NP | 4740 ± 80 | 0.6 ± 0.1 | ||
| Syringe 24 h (−20 °C) | 99.2 ± 0.8 * | 0.23 * | 72 ± 2 | 99.1 ± 0.3 | 889.8 | 0.2 | 4400 ± 200 | 0.8 ± 0.1 | ||
| Vial leftover 24 h (2–8 °C) | 91.6 ± 0.1 | 0.22 | 65 ± 3 | 99.5 ± 0.3 | 887.1 | 0.1 | 4500 ± 700 | 0.5 ± 0.2 | ||
| Parameters | Stress Test | Z-Average (nm) | PDI | |
|---|---|---|---|---|
| Vaccines | ||||
| Pfizer-BioNTech (Comirnaty™) | Unexpired | 88.7 ± 0.9 | 0.20 ± 0.02 | |
| Manual shaking | 116 ± 8 | 0.38 ± 0.02 | ||
| Manual shaking (30 days at 4 °C) | 112 ± 1 | 0.39 ± 0.01 | ||
| Vortex vibration | 96 ± 1 | 0.26 ± 0.01 | ||
| Expired | 157 ± 6 | 0.71 ± 0.06 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermosilla, J.; Alonso-García, A.; Salmerón-García, A.; Cabeza-Barrera, J.; Medina-Castillo, A.L.; Pérez-Robles, R.; Navas, N. Analysing the In-Use Stability of mRNA-LNP COVID-19 Vaccines Comirnaty™ (Pfizer) and Spikevax™ (Moderna): A Comparative Study of the Particulate. Vaccines 2023, 11, 1635. https://doi.org/10.3390/vaccines11111635
Hermosilla J, Alonso-García A, Salmerón-García A, Cabeza-Barrera J, Medina-Castillo AL, Pérez-Robles R, Navas N. Analysing the In-Use Stability of mRNA-LNP COVID-19 Vaccines Comirnaty™ (Pfizer) and Spikevax™ (Moderna): A Comparative Study of the Particulate. Vaccines. 2023; 11(11):1635. https://doi.org/10.3390/vaccines11111635
Chicago/Turabian StyleHermosilla, Jesús, Airan Alonso-García, Antonio Salmerón-García, José Cabeza-Barrera, Antonio L. Medina-Castillo, Raquel Pérez-Robles, and Natalia Navas. 2023. "Analysing the In-Use Stability of mRNA-LNP COVID-19 Vaccines Comirnaty™ (Pfizer) and Spikevax™ (Moderna): A Comparative Study of the Particulate" Vaccines 11, no. 11: 1635. https://doi.org/10.3390/vaccines11111635