The Dynamics of Changes in the Concentration of IgG against the S1 Subunit in Polish Healthcare Workers in the Period from 1 to 12 Months after Injection, Including Four COVID-19 Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants Group Characteristics
2.2. IgG Anti-SARS-CoV-2 Assays
2.3. Statistical Analysis
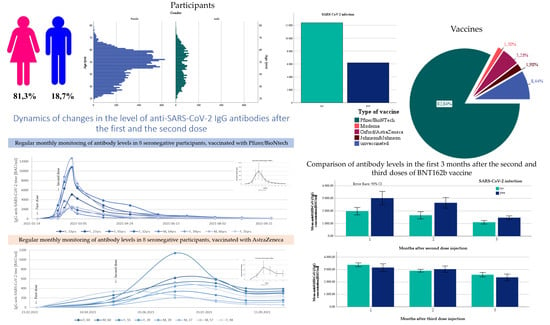
3. Results
3.1. Characteristics of Unvaccinated Participants
3.2. Comparison of Antibody Titers among Vaccines
3.3. Comparison of the Differential Concentration of Antibodies in Fully Vaccinated Subjects 6 Months after Injection
- Group 1—Vaccinated (two doses) more than 6 months before the antibody measurement, low antibodies level (<2500), regardless of the declaration of COVID-19 prior infection (N = 9181). In this group, we observed a systematic decrease in IgG concentration, which was a natural consequence of the lack of subsequent immunization;
- Group 2—Vaccinated (two doses) more than 6 months before the antibody measurement, high antibodies level (>2500), and no declaration of COVID-19 prior to infections (N = 190). In this group, we observed a significant increase in IgG concentration, probably a consequence of an unconscious (asymptomatic) SARS-CoV-2 infection;
- Group 3—Vaccinated (three doses), regardless of the declaration of COVID-19 prior to infection and antibodies level (N = 3191). Similar to the second group, we noted a significant increase in IgG levels as a result of the third injection.
3.4. Changes in Antibody Levels over 12 Months after Vaccination with BNT162b
3.5. Comparison of the Two Doses under 3 Months Vaccinated Group and the Three Doses under 3 Months Vaccinated Group
3.6. Changes in Antibody Levels over 10 Months after Vaccination with mRNA-1273
3.7. Changes in Antibody Levels over 8 Months after Vaccination with ChAdOx1 nCoV-2019
3.8. Changes in Antibody Levels over 8 Months after Vaccination with JNJ-78436735
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronawirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 3 February 2022).
- Website of the Ministry of Health-Poland. Available online: https://www.gov.pl/web/koronawirus (accessed on 3 February 2022).
- Zollner, A.; Watschinger, C.; Rossler, A.; Farcet, M.R.; Penner, A.; Bohm, V.; Kiechl, S.J.; Stampfel, G.; Hintenberger, R.; Tilg, H.; et al. B and T cell response to SARS-CoV-2 vaccination in health care professionals with and without previous COVID-19. EBioMedicine 2021, 70, 103539. [Google Scholar] [CrossRef] [PubMed]
- Summary of Product Characteristic-BNT162b2 Comirnaty Pfizer & BioNTech. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 3 February 2022).
- Summary of Product Characteristic-Spikevax Moderna. Available online: https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf (accessed on 3 February 2022).
- Summary of Product Characteristic-Vaxzevria AstraZeneca. Available online: https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-astrazeneca-product-information-approved-chmp-29-january-2021-pending-endorsement_en.pdf (accessed on 2 February 2022).
- Summary of Product Characteristic-JNJ78436735 Janssen/Johnson & Johnson. Available online: https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-janssen-epar-product-information_en.pdf (accessed on 3 February 2022).
- Cohn, B.A.; Cirillo, P.M.; Murphy, C.C.; Krigbaum, N.Y.; Wallace, A.W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2021, 375, eabm0620. [Google Scholar] [CrossRef]
- Reports of the Ministry of Health. Available online: https://www.gov.pl/web/koronawirus/wykaz-zarazen-koronawirusem-sars-cov-2 (accessed on 2 February 2022).
- Sternberg, A.; Naujokat, C. Structural features of coronavirus SARS-CoV-2 spike protein: Targets for vaccination. Life Sci. 2020, 257, 118056. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Tretyn, A.; Szczepanek, J.; Skorupa, M.; Jarkiewicz-Tretyn, J.; Sandomierz, D.; Dejewska, J.; Ciechanowska, K.; Jarkiewicz-Tretyn, A.; Koper, W.; Palgan, K. Differences in the concentration of anti-SARS-CoV-2 IgG antibodies post-COVID-19 recovery or post-vaccination. Cells 2021, 10, 1952. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021, 6, 104. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Kang, Y.M.; Minn, D.; Lim, J.; Lee, K.D.; Jo, D.H.; Choe, K.W.; Kim, M.J.; Kim, J.M.; Kim, K.N. Comparison of antibody response elicited by ChAdOx1 and BNT162b2 COVID-19 vaccine. J. Korean Med. Sci. 2021, 36, e311. [Google Scholar] [CrossRef]
- Szczepanek, J.; Skorupa, M.; Goroncy, A.; Jarkiewicz-Tretyn, J.; Wypych, A.; Sandomierz, D.; Jarkiewicz-Tretyn, A.; Dejewska, J.; Ciechanowska, K.; Pałgan, K.; et al. Anti-SARS-CoV-2 IgG against the S protein-comparison of 2 BNT162b2, mRNA-1273, ChAdOx1 nCoV-2019 and 3 Ad26.COV2.S vaccines. Vaccines 2022, 10, 90. [Google Scholar]
- Deming, M.E.; Lyke, K.E. A ‘mix and match’ approach to SARS-CoV-2 vaccination. Nat. Med. 2021, 27, 1510–1511. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Ukey, R.; Bruiners, N.; Mishra, H.; Mishra, P.K.; McCloskey, D.; Onyuka, A.; Chen, F.; Pinter, A.; Weiskopf, D.; Sette, A.; et al. Dichotomy between the humoral and cellular responses elicited by mRNA and adenoviral vector vaccines against SARS-CoV-2. BMC Med. 2021, 20, 1–7. [Google Scholar]
- Stuart, A.S.V.; Shaw, R.H.; Liu, X.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): A single-blind, randomised, phase 2, non-inferiority trial. Lancet 2022, 399, 36–49. [Google Scholar] [CrossRef]
- Lewis, D. Mix-and-match COVID vaccines: The case is growing, but questions remain. Nature 2021, 595, 344–345. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transpl. 2021, 21, 3990–4002. [Google Scholar] [CrossRef]
- Montoya, J.G.; Adams, A.E.; Bonetti, V.; Deng, S.; Link, N.A.; Pertsch, S.; Olson, K.; Li, M.; Dillon, E.C.; Frosch, D.L. Differences in IgG antibody responses following BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines. Microbiol. Spectr. 2021, 9, e0116221. [Google Scholar] [CrossRef]
- Al-Sadeq, D.W.; Shurrab, F.M.; Ismail, A.; Amanullah, F.H.; Thomas, S.; Aldewik, N.; Yassine, H.M.; Rahim, H.F.A.; Abu-Raddad, L.; Nasrallah, G.K. Comparison of antibody immune responses between BNT162b2 and mRNA-1273 SARS-CoV-2 vaccines in naïve and previously infected individuals. J. Travel. Med. 2021, 29, 8. [Google Scholar]
- Richards, N.E.; Keshavarz, B.; Workman, L.J.; Nelson, M.R.; Platts-Mills, T.A.E.; Wilson, J.M. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs. the mRNA-1273 vaccine. JAMA Netw. Open 2021, 4, e2124331. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Flaxman, A.; Marchevsky, N.G.; Jenkin, D.; Aboagye, J.; Aley, P.K.; Angus, B.; Belij-Rammerstorfer, S.; Bibi, S.; Bittaye, M.; Cappuccini, F.; et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: A substudy of two randomised controlled trials (COV001 and COV002). Lancet 2021, 398, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021, 11, 4583. [Google Scholar] [CrossRef]
- Brisotto, G.; Muraro, E.; Montico, M.; Corso, C.; Evangelista, C.; Casarotto, M.; Caffau, C.; Vettori, R.; Cozzi, M.R.; Zanussi, S.; et al. IgG antibodies against SARS-CoV-2 decay but persist 4 months after vaccination in a cohort of healthcare workers. Clin. Chim. Acta 2021, 523, 476–482. [Google Scholar] [CrossRef]
- Mishra, S.K.; Pradhan, S.K.; Pati, S.; Sahu, S.; Nanda, R.K. Waning of anti-spike antibodies in AZD1222 (ChAdOx1) vaccinated healthcare providers: A prospective longitudinal study. Cureus 2021, 13, e19879. [Google Scholar] [CrossRef]
- Xiao, K.; Yang, H.; Liu, B.; Pang, X.; Du, J.; Liu, M.; Liu, Y.; Jing, X.; Chen, J.; Deng, S.; et al. Antibodies can last for more than 1 year after SARS-CoV-2 infection: A follow-up study from survivors of COVID-19. Front. Med. 2021, 8, 684864. [Google Scholar] [CrossRef]
- Liu, Q.; Xiong, Q.; Mei, F.; Ma, C.; Zhang, Z.; Hu, B.; Xu, J.; Jiang, Y.; Zhan, F.; Zhou, S.; et al. Antibody neutralization to SARS-CoV-2 and variants after 1 year in Wuhan, China. Innov. 2022, 3, 100181. [Google Scholar] [CrossRef]
- Kucinskaite-Kodze, I.; Simanavicius, M.; Simaitis, A.; Zvirbliene, A. Persistence of SARS-CoV-2-specific antibodies for 13 months after infection. Viruses 2021, 13, 2313. [Google Scholar] [CrossRef]
- Ali, H.; Alahmad, B.; Al-Shammari, A.A.; Alterki, A.; Hammad, M.; Cherian, P.; Alkhairi, I.; Sindhu, S.; Thanaraj, T.A.; Mohammad, A.; et al. Previous COVID-19 infection and antibody levels after vaccination. Front. Public Health 2021, 9, 778243. [Google Scholar] [CrossRef]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, 1516.e7–1516.e14. [Google Scholar] [CrossRef]
- Angyal, A.; Longet, S.; Moore, S.C.; Payne, R.P.; Harding, A.; Tipton, T.; Rongkard, P.; Ali, M.; Hering, L.M.; Meardon, N.; et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: A multicentre prospective cohort study. Lancet Microbe 2022, 3, e21–e31. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Gurevich, M. Humoral SARS-COV-2 IgG decay within 6 months in COVID-19 healthy vaccinees: The need for a booster vaccine dose? Eur. J. Intern. Med. 2021, 94, 105–107. [Google Scholar] [CrossRef]
- Van Gils1, M.J.; Lavell, A.H.A.; van der Straten, K.; Appelman, B.; Bontjer, I.; Poniman, M.; Burger, J.A.; Oomen, M.; Bouhuijs, J.H.; van Vught, L.A.; et al. Four SARS-CoV-2 vaccines induce quantitatively different antibody responses against SARS-CoV-2 variants. medRxiv 2022. preprint. [Google Scholar]
- Lustig, Y.; Gonen, T.; Melzer, L.; Gilboa, M.; Indenbaum, V.; Cohen, C.; Amit, S.; Jaber, H.; Doolman, R.; Asraf, K.; et al. Superior immunogenicity and effectiveness of the 3rd BNT162b2 vaccine dose. medRxiv 2022. preprint. [Google Scholar]
- Demonbreun, A.R.; Sancilio, A.; Vaught, L.A.; Reiser, N.L.; Pesce, L.; McNally, E.M.; McDade, T.W. Antibody titers before and after booster doses of SARS-CoV-2 mRNA vaccines in healthy adults. medRxiv 2022. preprint. [Google Scholar]
- Gluck, V.; Grobecker, S.; Kostler, J.; Tydykov, L.; Bertok, M.; Weidlich, T.; Gottwald, C.; Salzberger, B.; Wagner, R.; Zeman, F.; et al. Immunity after COVID-19 and vaccination: Follow-up study over 1 year among medical personnel. Infection 2021, 2021, 1–8. [Google Scholar] [CrossRef]







| Total | Unvaccinated | BNT162b2 | mRNA-1273 | ChAdOx1 nCoV-2019 | Ad26.COV2.S | ||
|---|---|---|---|---|---|---|---|
| Age (y) | Under 35 | 3337 (17.9%) | 383 (24.4%) | 2539 (16.5%) | 222 (22.8%) | 106 (37.9%) | 87 (23.6%) |
| 36–45 | 3720 (20.0%) | 354 (22.5%) | 3020 (19.6%) | 210 (21.6%) | 73 (26.1%) | 63 (17.1%) | |
| 46–55 | 6201 (33.3%) | 459 (29.2%) | 5247 (34.0%) | 291 (29.9%) | 59 (21.1%) | 145 (39.3%) | |
| 56–65 | 4622 (24.8%) | 341 (21.7%) | 3960 (25.7%) | 222 (22.8%) | 33 (11.8%) | 66 (17.9%) | |
| 66+ | 729 (3.9%) | 33 (2.1%) | 650 (4.2%) | 29 (3.0%) | 9 (3.2%) | 8 (2.2%) | |
| Gender | Female | 15,133 (81.3%) | 1308 (83.3%) | 12527 (81.3%) | 784 (80.5%) | 225 (80.4%) | 289 (78.3%) |
| Male | 3477 (18.7%) | 262 (16.7%) | 2890 (18.7%) | 190 (19.5%) | 55 (19.6%) | 80 (21.7%) | |
| COVID-19 status | No infection | 12,397 (66.6%) | 941 (60.1%) | 10550 (68.4%) | 553 (56.8%) | 156 (55.7%) | 194 (52.6%) |
| Before vaccination | 6188 (33.3%) | 621 (39.6%) | 4848 (31.4%) | 421 (43.2%) | 123 (43.9%) | 175 (47.4%) | |
| After vaccination | 575 (3.1%) | 0 (0.0%) | 554 (3.6%) | 13 (1.3%) | 0 (0.0%) | 8 (2.2%) | |
| Twice | 19 (0.1%) | 5 (0.3%) | 14 (0.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Potential | 266 (1.4%) | 15 (1.0%) | 221 (1.4%) | 16 (1.6%) | 11 (3.9%) | 3 (0.8%) | |
| Time after Vaccination | ≤3 Months | 3–6 Months | 6–9 Months | 9+ Months | Total Number of Outliers | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 Prior to Infection | No | Yes | No | Yes | No | Yes | No | Yes | ||
| BNT162b2 | Value | 7680 | 8622 | 1880 | 3442.4 | 2524 | 4091.2 | 19,200 | 12,625 | 982 |
| Number of observations | 5 | 8 | 99 | 42 | 679 | 146 | 1 | 2 | ||
| mRNA-1273 | Value | 6957.6 | 13,977.5 | 7680 | 4412.6 | - | - | - | - | 11 |
| Number of observations | 5 | 1 | 1 | 4 | 0 | 0 | - | - | ||
| Time after Vaccination | ≤2 Months | 2–4 Months | 4+ Months | Total Number of Outliers | ||||
|---|---|---|---|---|---|---|---|---|
| COVID-19 Prior to Infection | No | Yes | No | Yes | No | Yes | ||
| ChAdOx1 nCoV-2019 | Value | 1914.1 | 4117 | 2188.4 | 3011 | 3124.6 | 3484.6 | 57 |
| Number of observations | 3 | 1 | 2 | 5 | 33 | 13 | ||
| JNJ-78436735 | Value | 6441.8 | - | - | 3561.4 | - | 7268.4 | 8 |
| Number of observations | 2 | 0 | 0 | 5 | 0 | 1 | ||
| Group | Minimum | 1st Quartile | Median | Mean | 3rd Quartile | Maximum | SD |
|---|---|---|---|---|---|---|---|
| Overall (N = 941) | 3.2 | 3.2 | 13.96 | 105.42 | 94.75 | 7680 | 488.80 |
| No antibodies (≤35.2) | 3.2 | 3.2 | 3.2 | 6.959 | 5.71 | 35.09 | 7.44 |
| Antibodies detected (>35.2) | 35.87 | 69.01 | 119.30 | 247.62 | 230.93 | 7680 | 741.95 |
| Month | Pfizer/BioNTech (BNT162b2) | Moderna (mRNA-1273) | Oxford/AstraZeneca (ChAdOx1 nCoV-2019) | Janssen/Johnson & Johnson (Ad26.COV2.S.) | ||||
|---|---|---|---|---|---|---|---|---|
| SARS-CoV-2 Infection | ||||||||
| No | Yes | No | Yes | No | Yes | No | Yes | |
| Mean Anti-SARS-CoV-2 IgG Concentration (BAU/mL) SD; 95%CI | ||||||||
| 1 | 3004.96 1858.58; 3421.26-2588.66 | 4358.83 3386.95; 5274.45–3443.21 | 4405.68 2504.75; 5997.13–2814.24 | 8079.23 3690.02; 11491.93–4666.53 | 706.5 673.53; 882.02–530.98 | 1065.56 818.68; 1308.68–822.44 | 1022.15 1807.59; 1643.08-401.22 | 2085.63 1448.83; 2697.42–1473.84 |
| 2 | 2067.00 1720.81; 2440.44–1693.56 | 3022.72 2601.41; 3629.67–2415.77 | 3537.75 2779.49; 7960.54–885.04 | 4398.72 2650.63; 6179.43–2618 | 526.37 448.52; 725.23–327.51 | 1136.3 856.56; 1497.99–774.61 | 1208.41 1789; 1813.72–603.1 | 2300.33 2385.00; 3225.14–1375.52 |
| 3 | 1342.04 1105; 1513.49–1170.59 | 1701.88 1111.18; 1876.49–1527.27 | 1643.6 1492.43; 2505.31–781.89 | 2551.25 1502.58; 3459.25–1643.25 | 287.26 249.52; 362.22–212.3 | 790.46 709.42; 986–594.91 | 720.98 957.9; 1135.21–306.75 | 959.45 852.71; 1290.1–628.8 |
| 4 | 975.90 919.26; 1108.88–842.92 | 1590.61 1463.17; 1822.78–1358.44 | 1460.96 968.00; 1748.42–1173.5 | 1230.23 702.04; 1568.6–891.86 | 271.72 279.64; 326.93–216.52 | 586.75 582.35; 716.35–457.16 | 745.59 925.35; 1041.53–449.64 | 1120.15 1565.49; 1666.37–573.92 |
| 5 | 627.01 688.93; 686.19–567.83 | 1180.34 1375.07; 1323.06–1037.62 | 1241.54 800.07; 1725.02–758.06 | 1893.14 1715.2; 2719.84–1066.44 | 328.80 * 59.48; 424.49–233.11 | 893.36 * 1671.5; 1283.35–503.37 | 634.46 * 940.99; 985.83–283.09 | 804.31 * 1037.53; 1178.38–430.24 |
| 6 | 543.20 722.77; 600.77–485.64 | 871.58 761.17; 947.56–795.61 | 1038.70 1095.82; 1537.52–539.89 | 1359.86 1334.91; 1937.12–782.6 | 1002.52 2177.32; 1419.83–585.2 | 855.43 1241.35; 1181.82–529.03 | 1080.98 1335.62; 1820.62–341.34 | 1345.10 1811.25; 2390.88–299.32 |
| 7 | 483.31 875.3; 535.72–430.9 | 811.35 955.99; 885.85–736.85 | 1120.12 1612.27; 1834.97–405.28 | 760.04 338.71; 955.6–564.47 | 1384.63 2247.99; 1799.89–969.36 | 1402.78 2030.03; 1886.83–918.74 | 1535.15 1575.86; 2662.46–407.85 | 790.33 701.39; 1195.29–385.36 |
| 8 | 328.62 613.31; 352.95–304.3 | 707.54 896.02; 762.81–652.27 | 1052.66 1242.57; 1691.53–413.79 | 691.53 474.96; 1447.29–64.24 | 348.94 468.06; 781.82–83.94 | 422.00 197.30; 586.95–257.05 | 1671.23 1111.52; 3439.9–97.45 | |
| 9 | 753.22 * 1419.24; 811.01–695.44 | 1109.24 * 1698.73; 1217.4–1001.08 | 2294.25 * 1940.98; 5382.78–794.28 | 908.6 * 695.57; 1772.26–44.94 | ||||
| 10 | 1952.48 2126.39; 2061.6–1843.35 | 2155.8 2271.71; 2346.77–1964.83 | 242.95 60.6; 787.41–301.51 | 1374.9 236.32; 3498.1–748.31 | ||||
| 11 | 2549.28 2242.13; 2662.16–2436.4 | 2284.43 2150.25; 2490.67–2078.19 | ||||||
| 12 | 2391.13 3100.24; 7324.3–2542.05 | |||||||
| Group | Minimum | 1st Quartile | Median | Mean | 3rd Quartile | Maximum | SD | |
|---|---|---|---|---|---|---|---|---|
| Healthy vaccinated (2 dose) more than 6 months before the antibody measurement | Overall (N = 6620) | 3.2 | 101.4 | 185.2 | 424.6 | 354.1 | 7730 | 911.40 |
| Low antibody level (≤2500) | 3.2 | 95.85 | 169.85 | 275.08 | 306.30 | 2473.60 | 337.91 | |
| High antibody level (>2500) | 2539 | 3763 | 4412 | 5062 | 7159 | 7730 | 1793.01 | |
| Vaccinated (3 dose), overall | 3.2 | 1490 | 2405.80 | 3007.80 | 3971.6 | 19,200 | 2161.04 | |
| Factors and Levels | BNT162b2 | mRNA-1273 | |||
|---|---|---|---|---|---|
| Mean * (Number of Observations) | 95% CI | Mean * (Number of Observations) | 95% CI | ||
| COVID-19 | no | 1216.062 (9687) | (1176.006, 1256.118) | 1375.350 (150) | (967.256, 1783.444) |
| yes | 1478.892 (4568) | (1415.960, 1541.824) | 1571.633 (112) | (1238.939, 1904.328) | |
| Time after vaccination | ≤3 months | 1772.822 (948) | (1678.493, 1867.151) | 2170.207 (121) | (1573.818, 2766.596) |
| 3–6 months | 601.301 (3461) | (582.563, 620.039) | 1050.766 (107) | (889.980, 1211.552) | |
| 6–9 months | 626.441 (7909) | (607.381, 645.500) | 1224.907 (34) | (727.943, 1721.871) | |
| 9+ months | 2384.526 (1937) | (2274.893, 2494.159) | - | - | |
| Time after vaccination and COVID-19 infection | ≤3 months, no | 1517.258 (512) | (1401.604, 1632.913) | 2149.050 (72) | (891.700, 3406.400) |
| ≤3 months, yes | 2034.188 (436) | (1880.238, 2188.138) | 2240.479 (49) | (1873.151, 2607.806) | |
| 3–6 months, no | 399.911 (2109) | (384.389, 415.432) | 1065.031 (55) | (796.358, 1333.704) | |
| 3–6 months, yes | 803.615 (1352) | (769.536, 837.694) | 1041.315 (52) | (860.521, 1222.110) | |
| 6–9 months, no | 407.1315 (5546) | (393.041, 421.222) | 1044.596 (23) | (617.441, 1471.751) | |
| 6–9 months, yes | 845.875 (2363) | (810.85, 880.900) | 1445.786 (11) | (555.032, 2336.539) | |
| 9+ months, no | 2537.310 (1520) | (2431.067, 2643.552) | - | - | |
| 9+ months, yes | 2237.735 (417) | (2046.920, 2428.549) | - | - | |
| Group | Mean | Lower CI | Upper CI |
|---|---|---|---|
| 1 | 374.50 | 366.35 | 382.64 |
| 2 | 5034.11 | 4777.52 | 5290.70 |
| 3 | 3007.76 | 2932.75 | 3082.77 |
| Factors and Levels | ChAdOx1 nCoV-2019 | JNJ-78436735 | |||
|---|---|---|---|---|---|
| Mean * (Number of Observations) | 95% CI | Mean * (Number of Observations) | 95% CI | ||
| COVID-19 | no | 382.500 (507) | (346.726, 418.274) | 960.268 (192) | (765.939, 1154.597) |
| yes | 706.674 (395) | (648.005, 765.343) | 1099.011 (169) | (936.426, 1261.595) | |
| Time after vaccination | ≤2 months | 702.293 (245) | (631.245, 773.340) | 1337.550 (172) | (1090.454, 1584.645) |
| 2–4 months | 413.318 (336) | (373.365, 453.27) | 688.845 (131) | (547.739, 829.95) | |
| 4+ months | 519.249 (321) | (455.447, 583.051) | 1058.729 (58) | (784.584, 1332.875) | |
| Time after vaccination and COVID-19 infection | ≤2 months, no | 473.535 (123) | (404.932, 542.138) | 891.668 (92) | (612.647, 1170.689) |
| ≤2 months, yes | 938.1125 (122) | (807.835, 1068.390) | 1791.968 (80) | (1402.612, 2181.325) | |
| 2–4 months, no | 269.658 (188) | (238.669, 300.647) | 704.624 (70) | (492.279, 916.969) | |
| 2–4 months, yes | 556.898 (148) | (483.554, 630.242) | 672.644 (61) | (488.824, 856.464) | |
| 4+ months, no | 409.152 (196) | (328.539, 489.765) | 1293.385 (30) | (810.096, 1776.675) | |
| 4+ months, yes | 634.559 (125) | (536.503, 732.615) | 842.969 (28) | (596.795, 1089.142) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skorupa, M.; Szczepanek, J.; Goroncy, A.; Jarkiewicz-Tretyn, J.; Ptaszyńska, B.; Rajewski, P.; Koper, W.; Pałgan, K.; Tretyn, A. The Dynamics of Changes in the Concentration of IgG against the S1 Subunit in Polish Healthcare Workers in the Period from 1 to 12 Months after Injection, Including Four COVID-19 Vaccines. Vaccines 2022, 10, 506. https://doi.org/10.3390/vaccines10040506
Skorupa M, Szczepanek J, Goroncy A, Jarkiewicz-Tretyn J, Ptaszyńska B, Rajewski P, Koper W, Pałgan K, Tretyn A. The Dynamics of Changes in the Concentration of IgG against the S1 Subunit in Polish Healthcare Workers in the Period from 1 to 12 Months after Injection, Including Four COVID-19 Vaccines. Vaccines. 2022; 10(4):506. https://doi.org/10.3390/vaccines10040506
Chicago/Turabian StyleSkorupa, Monika, Joanna Szczepanek, Agnieszka Goroncy, Joanna Jarkiewicz-Tretyn, Barbara Ptaszyńska, Paweł Rajewski, Wojciech Koper, Krzysztof Pałgan, and Andrzej Tretyn. 2022. "The Dynamics of Changes in the Concentration of IgG against the S1 Subunit in Polish Healthcare Workers in the Period from 1 to 12 Months after Injection, Including Four COVID-19 Vaccines" Vaccines 10, no. 4: 506. https://doi.org/10.3390/vaccines10040506