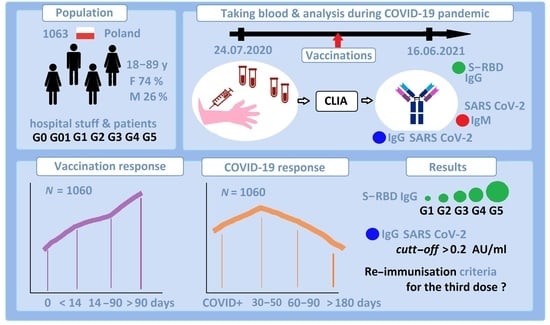
Systemic COVID-19 Vaccination Enhances the Humoral Immune Response after SARS-CoV-2 Infection: A Population Study from a Hospital in Poland Criteria for COVID-19 Reimmunization Are Needed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Statistical Analysis
4. Results
5. Discussion
5.1. Who Needs the Third Dose of the Vaccine?
5.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.; Mellinghoff, S.C.; Shamsrizi, P.; Addo, M.M.; Dahlke, C. Correlates of Vaccine-Induced Protection against SARS-CoV-2. Vaccines 2021, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Lucas, C.; Klein, J.; Sundaram, M.E.; Liu, F.; Wong, P.; Silva, J.; Mao, T.; Oh, J.E.; Mohanty, S.; Huang, J.; et al. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat. Med. 2021, 27, 1178–1186. [Google Scholar] [CrossRef]
- Dufloo, J.; Grzelak, L.; Staropoli, I.; Madec, Y.; Tondeur, L.; Anna, F.; Pelleau, S.; Wiedemann, A.; Planchais, C.; Buchrieser, J.; et al. Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies. Cell Rep. Med. 2021, 2, 100275. [Google Scholar] [CrossRef]
- Vicenti, I.; Gatti, F.; Scaggiante, R.; Boccuto, A.; Zago, D.; Basso, M.; Dragoni, F.; Parisi, S.G.; Zazzi, M. The second dose of the BNT162b2 mRNA vaccine does not boost SARS-CoV-2 neutralizing antibody response in previously infected subjects. Infection 2021. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse Functional Autoantibodies in Patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef]
- Becker, M.; Dulovic, A.; Junker, D.; Ruetalo, N.; Kaiser, P.D.; Pinilla, Y.T.; Heinzel, C.; Haering, J.; Traenkle, B.; Wagner, T.R.; et al. Immune response to SARS-CoV-2 variants of concern in vaccinated individuals. Nat. Commun. 2021, 12, 3109. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Kang, X.; Zhang, B.; Zheng, S.; Liu, B.; Yu, T.; Yang, F.; Wang, Q.; Miao, H. Plasma therapy cured a COVID-19 patient with long duration of viral shedding for 49 days: The clinical features, laboratory tests, plasma therapy, and implications for public health management. MedComm 2020, 1, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.G.; Hur, J.; Hong, K.S.; Lee, W.; Ahn, J.H. Prognostic Accuracy of the SIRS, qSOFA, and NEWS for Early Detection of Clinical Deterioration in SARS-CoV-2 Infected Patients. J. Korean Med. Sci. 2020, 35, e234. [Google Scholar] [CrossRef] [PubMed]
- Arvin, A.M.; Fink, K.; Schmid, M.A.; Cathcart, A.; Spreafico, R.; Havenar-Daughton, C.; Lanzavecchia, A.; Corti, D.; Virgin, H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020, 584, 353–363. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. COVID-19 convalescent plasma therapy and immunodeficiency. Transfus Clin. Biol. 2021, 28, 306–307. [Google Scholar] [CrossRef]
- Kow, C.S.; Hasan, S.S. Real-world effectiveness of BNT162b2 mRNA vaccine: A meta-analysis of large observational studies. Inflammopharmacology 2021, 29, 1075–1090. [Google Scholar] [CrossRef]
- Kontopoulou, K.; Ainatzoglou, A.; Nakas, C.T.; Ifantidou, A.; Goudi, G.; Antoniadou, E.; Adamopoulos, V.; Papadopoulos, N.; Papazisis, G. Second dose of the BNT162b2 mRNA vaccine: Value of timely administration but questionable necessity among the seropositive. Vaccine 2021, 39, 5078–5081. [Google Scholar] [CrossRef]
- Polewska, K.; Tylicki, P.; Biedunkiewicz, B.; Rucińska, A.; Szydłowska, A.; Kubanek, A.; Rosenberg, I.; Rodak, S.; Ślizień, W.; Renke, M.; et al. Safety and Tolerability of the BNT162b2 mRNA COVID-19 Vaccine in Dialyzed Patients. COViNEPH Project. Medicina 2021, 57, 732. [Google Scholar] [CrossRef]
- Perry, C.; Luttwak, E.; Balaban, R.; Shefer, G.; Morales, M.M.; Aharon, A.; Tabib, Y.; Cohen, Y.C.; Benyamini, N.; Beyar-Katz, O.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021, 5, 3053–3061. [Google Scholar] [CrossRef]
- Stumpf, J.; Tonnus, W.; Paliege, A.; Rettig, R.; Steglich, A.; Gembardt, F.; Kessel, F.; Kröger, H.; Arndt, P.; Sradnick, J.; et al. Cellular and Humoral Immune Responses after Three Doses of BNT162b2 mRNA SARS-Cov-2 Vaccine in Kidney Transplant. Transplantation 2021, 105, e267–e269. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Tang, P.; Malek, J.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Pfizer-BioNTech mRNA BNT162b2 COVID-19 vaccine protection against variants of concern after one versus two doses. J. Travel Med. 2021, 28. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Li, T.-D.; Zheng, S.-F.; Su, Y.-Y.; Li, Z.-Y.; Liu, W.; Yu, F.; Ge, S.-X.; Zou, Q.-D.; Yuan, Q.; et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur. Respir. J. 2020, 56, 200076. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, F.; Buonfrate, D.; Silva, R.; Martini, D.; Bisoffi, Z.; Piubelli, C.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody response in individuals infected with SARS-CoV-2 early after the first dose of the BNT162b2 mRNA vaccine. J. Infect. 2021, 84, 94–118. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Merzon, E.; Schaffer, A.A.; Shenhar, Y.; Green, I.; Golan-Cohen, A.; Ruppin, E.; Magen, E.; Vinker, S. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort. medRxiv 2021. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Vita, R.; Peters, B.; Crotty, S.; Weiskopf, D.; Sette, A. SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host Microbe 2021, 29, 1076–1092. [Google Scholar] [CrossRef]
- Liao, B.; Chen, Z.; Zheng, P.; Li, L.; Zhuo, J.; Li, F.; Li, S.; Chen, D.; Wen, C.; Cai, W.; et al. Detection of Anti-SARS-CoV-2-S2 IgG Is More Sensitive Than Anti-RBD IgG in Identifying Asymptomatic COVID-19 Patients. Front. Immunol. 2021, 12, 724763. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health-Eur. 2021, 10, 100208. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.-X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef]




| Groups | Participants | G0 | G01 | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|---|---|---|
| All | 1060 | 111 | 43 | 76 | 472 | 42 | 312 | 4 |
| % | 100 | 10.69 | 4.05 | 7.17 | 44.52 | 3.96 | 29.43 | 0.38 |
| 2020 | 499 (47.07%) | 51 | 14 | 30 | 215 | 7 | 179 | 3 |
| 2021 | 561 (52.92%) | 60 | 29 | 46 | 257 | 35 | 133 | 1 |
| Sex | ||||||||
| Female | 783 (73.87%) | 94 | 10 | 33 | 63 | 346 | 23 | 3 |
| Vaccinated | 594 (75.86%) | 0 | 0 | 0 | 347 | 23 | 221 | 3 |
| Unvaccinated | 189 (24.14%) | 93 | 33 | 63 | 0 | 0 | 0 | 0 |
| Male | 277 (26.13%) | 18 | 33 | 10 | 125 | 19 | 91 | 1 |
| Vaccinated | 236 (85.19%) | 0 | 0 | 0 | 125 | 19 | 91 | 1 |
| Unvaccinated | 41 (14.8%) | 18 | 10 | 13 | 0 | 0 | 0 | 0 |
| Age range | ||||||||
| <35 | 220 (20.75%) | 39 | 8 | 20 | 102 | 4 | 47 | 0 |
| 36–49 | 361 (34.06%) | 39 | 12 | 23 | 163 | 15 | 109 | 0 |
| >50 | 479 (45.18%) | 34 | 23 | 33 | 206 | 23 | 156 | 4 |
| COVID-19 test | ||||||||
| Negative | 671 (63.3%) | 111 | 43 | 0 | 471 | 35 | 11 | 0 |
| Positive | 389 (36.69%) | 0 | 0 | 76 | 1 | 7 | 301 | 4 |
| COVID-19 response | ||||||||
| No | 662 (62.45%) | 111 | 43 | 0 | 470 | 35 | 2 | 0 |
| 30–50 days | 18 (1.69%) | 0 | 0 | 13 | 1 | 2 | 1 | 2 |
| 60–90 days | 81 (7.64%) | 0 | 0 | 18 | 0 | 3 | 58 | 2 |
| >180 days | 299 (28.2%) | 0 | 0 | 45 | 1 | 0 | 251 | 0 |
| Vaccinated | ||||||||
| No | 233 (21.79%) | 111 | 43 | 76 | 0 | 0 | 0 | 0 |
| Yes | 827 (78.01%) | 0 | 0 | 0 | 472 | 24 | 312 | 4 |
| Comirnaty | 787 (74.2%) | 0 | 0 | 0 | 472 | 0 | 312 | 4 |
| AstraZeneca | 21 (1.98%) | 0 | 0 | 0 | 0 | 21 | 0 | 0 |
| Moderna | 19 (1.79%) | 0 | 0 | 0 | 0 | 19 | 0 | 0 |
| J&J | 2 (0.19%) | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Vaccinated response | ||||||||
| No | 749 (70.66%) | 111 | 43 | 76 | 288 | 31 | 197 | 2 |
| <14 days | 58 (5.47%) | 0 | 0 | 0 | 28 | 4 | 26 | 0 |
| 14–90 days | 183 (17.3%) | 0 | 0 | 0 | 111 | 0 | 64 | 1 |
| >90 days | 70 (6.6%) | 0 | 0 | 0 | 45 | 0 | 25 | 1 |
| IgG (AU/mL) | 1060 | |||||||
| <1.0 | 685 (64.62%) | 111 | 19 | 41 | 420 | 27 | 192 | 3 |
| >0.2 | 375 (35.37%) | 0 | 43 | 42 | 103 | 19 | 167 | 1 |
| >1.0 | 247 (23.30%) | 0 | 24 | 35 | 52 | 15 | 120 | 1 |
| IgM (AU/mL) | 1060 | |||||||
| <1.0 | 879 (82.92%) | 103 | 33 | 65 | 397 | 36 | 242 | 3 |
| >1.0 | 181 (17.07%) | 18 | 11 | 75 | 6 | 70 | 1 | |
| S-RBD IgG (AU/mL) | 546 | |||||||
| <1.0 | 81 (7.64%) | 31 | 2 | 6 | 31 | 4 | 7 | 0 |
| >1.0 | 465 (43.86%) | 20 | 22 | 33 | 220 | 21 | 147 | 2 |
| >50 | 295 (27.83%) | 0 | 3 | 11 | 154 | 8 | 117 | 2 |
| >100 | 246 (23.20%) | 0 | 3 | 8 | 126 | 6 | 101 | 2 |
| >500 | 57 (5.38%) | 0 | 0 | 1 | 14 | 4 | 36 | 2 |
| >1000 | 38 (3.58%) | 0 | 0 | 0 | 10 | 3 | 23 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosiorek, P.; Kazberuk, D.E.; Hryniewicz, A.; Milewski, R.; Stróż, S.; Stasiak-Barmuta, A. Systemic COVID-19 Vaccination Enhances the Humoral Immune Response after SARS-CoV-2 Infection: A Population Study from a Hospital in Poland Criteria for COVID-19 Reimmunization Are Needed. Vaccines 2022, 10, 334. https://doi.org/10.3390/vaccines10020334
Kosiorek P, Kazberuk DE, Hryniewicz A, Milewski R, Stróż S, Stasiak-Barmuta A. Systemic COVID-19 Vaccination Enhances the Humoral Immune Response after SARS-CoV-2 Infection: A Population Study from a Hospital in Poland Criteria for COVID-19 Reimmunization Are Needed. Vaccines. 2022; 10(2):334. https://doi.org/10.3390/vaccines10020334
Chicago/Turabian StyleKosiorek, Piotr, Dorota Elżbieta Kazberuk, Anna Hryniewicz, Robert Milewski, Samuel Stróż, and Anna Stasiak-Barmuta. 2022. "Systemic COVID-19 Vaccination Enhances the Humoral Immune Response after SARS-CoV-2 Infection: A Population Study from a Hospital in Poland Criteria for COVID-19 Reimmunization Are Needed" Vaccines 10, no. 2: 334. https://doi.org/10.3390/vaccines10020334