Long-Term Antibody Response against SARS-CoV-2 in Health Care Workers: Effectiveness of Homologous and Heterologous Regimens and Their Relation to Systemic Vaccine-Associated Symptoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. SARS-CoV-2 Antibody Measurement
2.3. Statistical Analysis
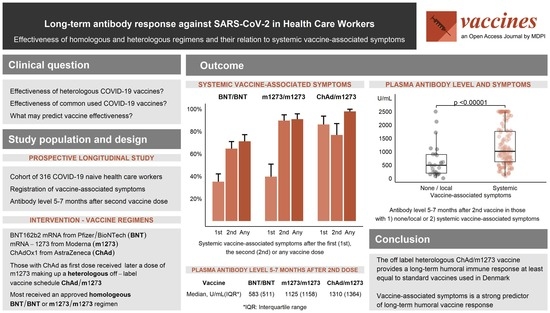
3. Results
3.1. Study Participants and Vaccine Regiments
3.2. Vaccine-Associated Symptoms
3.3. Early and Late Antibody Level According to Vaccine Regimen
3.4. Vaccine-Associated Symptoms Predict Late Antibody Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VAS | Vaccine-associated symptoms |
| BNT | BNT162b2 mRNA vaccine from Pfizer/BioNTech |
| ChAd | ChAdOx1 nCoV-19 from AstraZeneca |
| m1273 | mRNA-1273 vaccine from Moderna |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| HCW | Health care workers |
| VITT | Vaccine-Induced Immune Thrombosis and Thrombocytopenia |
| COVID-19 | Coronavirus disease 2019 |
References
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.-D.; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population based cohort study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef] [PubMed]
- Gram, M.A.; Nielsen, J.; Schelde, A.B.; Nielsen, K.F.; Moustsen-Helms, I.R.; Sørensen, A.K.B.; Valentiner-Branth, P.; Emborg, H.-D. Vaccine effectiveness against SARS-CoV-2 infection, hospitalization, and death when combining a first dose ChAdOx1 vaccine with a subsequent mRNA vaccine in Denmark: A nationwide population-based cohort study. PLoS Med. 2021, 18, e1003874. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Sester, M.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, S.M.; Endres-Dighe, S.M.; Angulo, F.J.; Srivastava, A.; Nguyen, J.L.; Khan, F.; Martin, C.; Swerdlow, D.L.; McLaughlin, J.M.; Ubaka-Blackmore, N.; et al. COVID-19 Vaccine Effectiveness: A Review of the First 6 Months of COVID-19 Vaccine Availability (1 January–30 June 2021). Vaccines 2022, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Kontou, P.I.; Braliou, G.G.; Dimou, N.L.; Nikolopoulos, G.; Bagos, P.G. Antibody Tests in Detecting SARS-CoV-2 Infection: A Meta-Analysis. Diagnostics 2020, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, B.; Giglio, R.V.; Vidali, M.; Scazzone, C.; Bivona, G.; Gambino, C.M.; Ciaccio, A.M.; Agnello, L.; Ciaccio, M. Evaluation of Anti-SARS-Cov-2 S-RBD IgG Antibodies after COVID-19 mRNA BNT162b2 Vaccine. Diagnostics 2021, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, P.; Maruste, R.; Kärner, J.; et al. Declined antibody responses to COVID-19 mRNA vaccine within first three months. medRxiv 2021. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fukunaga, A.; Tanaka, A.; Takeuchi, J.S.; Inoue, Y.; Kimura, M.; Maeda, K.; Ueda, G.; Mizoue, T.; Ujiie, M.; et al. Association between reactogenicity and SARS-CoV-2 antibodies after the second dose of the BNT162b2 COVID-19 vaccine. Vaccine 2022, 40, 1924–1927. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.A.; Laing, E.D.; Olsen, C.H.; Goguet, E.; Moser, M.; Jackson-Thompson, B.M.; Samuels, E.C.; Pollett, S.D.; Tribble, D.R.; Davies, J.; et al. Adverse Effects and Antibody Titers in Response to the BNT162b2 mRNA COVID-19 Vaccine in a Prospective Study of Healthcare Workers. Open Forum. Infect. Dis. 2022, 9, ofab575. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Moon, J.Y.; Lee, S.K.; Lee, H.; Moon, S.; Chung, S.J.; Yeo, Y.; Park, T.S.; Park, D.W.; Kim, T.-H.; et al. Anti-SARS-CoV-2 Spike Protein RBD Antibody Levels After Receiving a Second Dose of ChAdOx1 nCov-19 (AZD1222) Vaccine in Healthcare Workers: Lack of Association With Age, Sex, Obesity, and Adverse Reactions. Front. Immunol. 2021, 12, 779212. [Google Scholar] [CrossRef]
- Factsheet Elecsys® Anti-SARS-CoV-2 S. Available online: https://diagnostics.roche.com/content/dam/diagnostics/Blueprint/en/pdf/cps/factsheet-elecsys-anti-sars-cov-2-s-mc--05522.pdf (accessed on 15 September 2022).
- Lin, T.Y.; Hung, N.K.; Hung, S.C. Association of Reactogenicity with Immunogenicity of the ChAdOx1 nCoV-19 Vaccine in Patients Undergoing Hemodialysis. Vaccines 2022, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Choi, S.Y.; Lee, Y.M.; Kim, H.W. Neutralizing Antibody Response, Safety, and Efficacy of mRNA COVID-19 Vaccines in Pediatric Patients with Inflammatory Bowel Disease: A Prospective Multicenter Case-Control Study. Vaccines 2022, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Toapanta-Yanchapaxi, L.; Chiquete, E.; Ávila-Rojo, E.; López-Yánez, S.; Luna Del Villar Velasco, S.; Rivera Monroy, S.; López Gómez, T.; Andrés Aguilar, J.B.; Balcázar Antonio, D.F.; Alcaraz-Fuerte, C.; et al. Humoral response to different SARS-CoV-2 vaccines in orthotopic liver transplant recipients. Vaccines 2022, 40, 5621–5630. [Google Scholar] [CrossRef] [PubMed]
- Amraotkar, A.R.; Bushau-Sprinkle, A.M.; Keith, R.J.; Hamorsky, K.T.; Palmer, K.E.; Gao, H.; Rai, S.N.; Bhatnagar, A. Pre-Existing Comorbidities Diminish the Likelihood of Seropositivity after SARS-CoV-2 Vaccination. Vaccines 2022, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Karalis, V.; Ntanasis-Stathopoulos, I.; Evangelakou, Z.; Gavriatopoulou, M.; Manola, M.S.; Malandrakis, P.; Gianniou, D.D.; Kastritis, E.; Trougakos, I.P.; et al. Comparison of Neutralizing Antibody Responses at 6 Months Post Vaccination with BNT162b2 and AZD1222. Biomedicines. 2022, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lim, S.Y.; Park, S.; Kwon, J.S.; Bae, S.; Park, J.Y.; Cha, H.H.; Seo, M.H.; Lee, H.J.; Lee, N.; et al. Immune Responses to the ChAdOx1 nCoV-19 and BNT162b2 Vaccines and to Natural Coronavirus Disease 2019 Infections Over a 3-Month Period. J. Infect. Dis. 2022, 225, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.H.; Chang, S.Y.; Lin, P.H.; Hsieh, M.J.; Chang, H.H.; Cheng, C.Y.; Yang, H.C.; Pan, C.F.; Ieong, S.M.; Chao, T.L.; et al. Immune response and safety of heterologous ChAdOx1-nCoV-19/mRNA-1273 vaccination compared with homologous ChAdOx1-nCoV-19 or homologous mRNA-1273 vaccination. J. Formos. Med. Assoc. 2022, 121, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, B.; Godono, A.; Comoretto, R.I.; Casabona, E.; Curoso, G.; Leone, M.V.; Milanesio, N.; Mirra, I.; Montrucchio, G.; Pittaluga, F. Immune Response of a Heterologous mRNA-1273 Second-Dose Immunization after a First Dose of ChadOx1 against SARS-CoV-2: A Cross-Sectional Study. Vaccines 2022, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Kaku, C.I.; Champney, E.R.; Normark, J.; Garcia, M.; Johnson, C.E.; Ahlm, C.; Christ, W.; Sakharkar, M.; Ackerman, M.E.; Klingström, J.; et al. Broad anti-SARS-CoV-2 antibody immunity induced by heterologous ChAdOx1/mRNA-1273 vaccination. Science 2022, 375, 1041–1047. [Google Scholar] [CrossRef] [PubMed]




| BNT/BNT | m1273/m1273 | ChAd/m1273 | p-Value 1 | |
|---|---|---|---|---|
| Answers on questionnaire, n | 209 | 82 | 46 | |
| PCR-confirmed SARS-CoV-2 infection prior to first vaccine dose, n (%) (PP) | 12 (6%) | 6 (7%) | 3 (7%) | 0.88 |
| PCR-confirmed SARS-CoV-2 during follow up, n | 1 | 0 | 0 | |
| Study cohort excluding individuals with PCR-confirmed SARS-CoV-2 infection prior to first vaccine dose | ||||
| Demographics | ||||
| HWCs (N) | 197 | 76 | 43 | |
| Sex: female (%) | 185 (94%) | 70 (92%) | 39 (90%) | 0.70 |
| Age: median (range), years | 48 (19–67) | 50 (21–67) | 45 (24–64) | 0.56 * |
| Preexisting co-morbidity | 48 (24%) | 23 (30%) | 9 (21%) | 0.47 |
| Vaccine-associated symptoms | ||||
| Systemic symptoms | ||||
| After the first dose | 69 (35%) | 30 (39%) | 37 (86%) | <0.0001 |
| After the second dose | 127 (64%) | 68 (89%) | 33 (77%) | <0.001 |
| After any dose | 140 (71%) | 69 (91%) | 42 (98%) | <0.0000001 |
| Local symptoms | ||||
| After the first dose | 162 (82%) | 71 (93%) | 36 (84%) | 0.06 |
| After the second dose | 137 (70%) | 71 (93%) | 33 (80%) | <0.001 |
| After any dose | 174(88%) | 75(99%) | 38(93%) | 0.02 |
| Any VAS after any dose | 191 (97%) | 76 (100%) | 43 (100%) | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kierkegaard, H.; Røge, B.T.; Nissen, A.; Madsen, J.S. Long-Term Antibody Response against SARS-CoV-2 in Health Care Workers: Effectiveness of Homologous and Heterologous Regimens and Their Relation to Systemic Vaccine-Associated Symptoms. Vaccines 2022, 10, 1599. https://doi.org/10.3390/vaccines10101599
Kierkegaard H, Røge BT, Nissen A, Madsen JS. Long-Term Antibody Response against SARS-CoV-2 in Health Care Workers: Effectiveness of Homologous and Heterologous Regimens and Their Relation to Systemic Vaccine-Associated Symptoms. Vaccines. 2022; 10(10):1599. https://doi.org/10.3390/vaccines10101599
Chicago/Turabian StyleKierkegaard, Helene, Birgit Thorup Røge, Amanda Nissen, and Jonna Skov Madsen. 2022. "Long-Term Antibody Response against SARS-CoV-2 in Health Care Workers: Effectiveness of Homologous and Heterologous Regimens and Their Relation to Systemic Vaccine-Associated Symptoms" Vaccines 10, no. 10: 1599. https://doi.org/10.3390/vaccines10101599