Early Senescence and Leukocyte Telomere Shortening in SCHIZOPHRENIA: A Role for Cytomegalovirus Infection?
Abstract
:1. Introduction
2. CMV Is a Major Driver of Immunosenescence
2.1. CMV and Leukocyte Telomere Shortening in Healthy Ageing
2.2. CMV Seropositivity and Inflammation
3. Telomere Length in Age-Associated Diseases
4. Infection by CMV, T. Gondii and other Pathogens in Schizophrenia
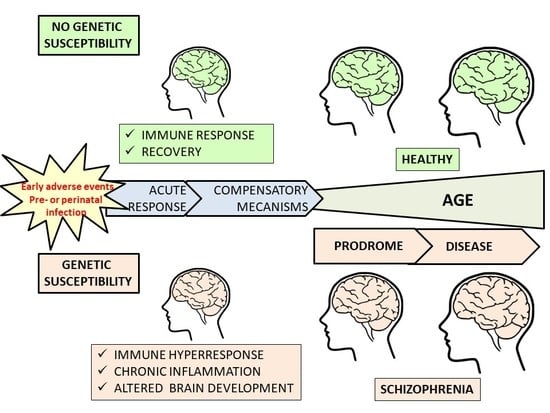
5. Schizophrenia, Inflammation, and Early Senescence
5.1. Schizophrenia and Inflammation
5.2. Leukocyte Telomere Shortening in Schizophrenia
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Lieberman, J.A.; First, M.B. Psychotic Disorders. N. Engl. J. Med. 2018, 379, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Prim. 2015, 1, 15067. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, A.L. Psychotic and Bipolar Disorders: Schizophrenia. FP Essent. 2017, 455, 11–17. [Google Scholar] [PubMed]
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [Green Version]
- Foley, C.; Corvin, A.; Nakagome, S. Genetics of Schizophrenia: Ready to Translate? Curr. Psychiatry Rep. 2017, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.L.; Herrera, L.; Gaspar, P.A.; Nieto, R.; Maturana, A.; Villar, M.J.; Salinas, V.; Silva, H. Shifting the focus toward rare variants in schizophrenia to close the gap from genotype to phenotype. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, F.; Liu, L.; Wang, L.; Chen, X. Genetic studies of schizophrenia: An update. Neurosci. Bull. 2015, 31, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.; Prata, D.P. What is the impact of genome-wide supported risk variants for schizophrenia and bipolar disorder on brain structure and function? A systematic review. Psychol. Med. 2015, 45, 2461–2480. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.R.; Howrigan, D.P.; Merico, D.; Thiruvahindrapuram, B.; Wu, W.; Greer, D.S.; Antaki, D.; Shetty, A.; Holmans, P.A.; Pinto, D.; et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat. Genet. 2017, 49, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ripke, S.; O’Dushlaine, C.; Chambert, K.; Moran, J.L.; Kähler, A.K.; Akterin, S.; Bergen, S.E.; Collins, A.L.; Crowley, J.J.; Fromer, M.; et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. 2013, 45, 1150–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, A. Imaging genetics of schizophrenia in the post-GWAS era. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Walton, N.M.; Yamada, H.; Kondo, Y.; Marek, G.J.; Tajinda, K. The impact of genetics on future drug discovery in schizophrenia. Expert Opin. Drug Discov. 2017, 12, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Ranlund, S.; Calafato, S.; Thygesen, J.H.; Lin, K.; Cahn, W.; Crespo-Facorro, B.; de Zwarte, S.M.C.; Díez, Á.; Di Forti, M.; Iyegbe, C.; et al. A polygenic risk score analysis of psychosis endophenotypes across brain functional, structural, and cognitive domains. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018, 177, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, N.; ban-Huard, C.; Godin, O.; Laouamri, H.; Jamain, S.; Attiba, D.; Delavest, M.; Lépine, J.P.; Le Corvoisier, P.; Houenou, J.; et al. Effects of Cumulative Herpesviridae and Toxoplasma gondii Infections on Cognitive Function in Healthy, Bipolar, and Schizophrenia Subjects. J. Clin. Psychiatry 2017, 78, e18–e27. [Google Scholar] [CrossRef] [PubMed]
- Fuglewicz, A.J.; Piotrowski, P.; Stodolak, A. Relationship between toxoplasmosis and schizophrenia: A review. Adv. Clin. Exp. Med. 2017, 26, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Gatov, E.; Rosella, L.; Chiu, M.; Kurdyak, P.A. Trends in standardized mortality among individuals with schizophrenia, 1993–2012: A population-based, repeated cross-sectional study. CMAJ 2017, 189, E1177–E1187. [Google Scholar] [CrossRef] [PubMed]
- Olfson, M.; Gerhard, T.; Huang, C.; Crystal, S.; Stroup, T.S. Premature Mortality among Adults with Schizophrenia in the United States. JAMA Psychiatry 2015, 72, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, P.; Gondek, T.M.; Krolicka-Deregowska, A.; Misiak, B.; Adamowski, T.; Kiejna, A. Causes of mortality in schizophrenia: An updated review of European studies. Psychiatr. Danub. 2017, 29, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Osby, U.; Westman, J.; Hallgren, J.; Gissler, M. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987–2010. Eur. J. Public Health 2016, 26, 867–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meesters, P.D.; Comijs, H.C.; Smit, J.H.; Eikelenboom, P.; de Han, L.; Beekman, A.T.; Stek, M.L. Mortality and Its Determinants in Late-Life Schizophrenia: A 5-Year Prospective Study in a Dutch Catchment Area. Am. J. Geriatr. Psychiatry 2016, 24, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Hjorthoj, C.; Sturup, A.E.; McGrath, J.J.; Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry 2017, 4, 295–301. [Google Scholar] [CrossRef]
- Islam, F.; Mulsant, B.H.; Voineskos, A.N.; Rajji, T.K. Brain-Derived Neurotrophic Factor Expression in Individuals with Schizophrenia and Healthy Aging: Testing the Accelerated Aging Hypothesis of Schizophrenia. Curr. Psychiatry Rep. 2017, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, B.; Kennedy, B.K. Accelerated aging in schizophrenia and related disorders: Future research. Schizophr. Res. 2018, 196, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Solana, R.; Tarazona, R.; Aiello, A.E.; Akbar, A.N.; Appay, V.; Beswick, M.; Bosch, J.A.; Campos, C.; Cantisán, S.; Cicin-Sain, L.; et al. CMV and Immunosenescence: From basics to clinics. Immun. Ageing 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Larbi, A.; Ozcelik, D.; Solana, R.; Gouttefangeas, C.; Attig, S.; Wikby, A.; Strindhall, J.; Franceschi, C.; Pawelec, G. Cytomegalovirus infection: A driving force in human T cell immunosenescence. Ann. N. Y. Acad. Sci. 2007, 1114, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Akbar, A.; Caruso, C.; Solana, R.; Grubeck-Loebenstein, B.; Wikby, A. Human immunosenescence: Is it infectious? Immunol. Rev. 2005, 205, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Hassouneh, F.; Lopez-Sejas, N.; Campos, C.; Sanchez-Correa, B.; Tarazona, R.; Pera, A.; Solana, R. Effect of Cytomegalovirus (CMV) and Ageing on T-Bet and Eomes Expression on T-Cell Subsets. Int. J. Mol. Sci. 2017, 18, 1391. [Google Scholar] [CrossRef] [PubMed]
- Pera, A.; Vasudev, A.; Tan, C.; Kared, H.; Solana, R.; Larbi, A. CMV induces expansion of highly polyfunctional CD4+ T cell subset coexpressing CD57 and CD154. J. Leukoc. Biol. 2017, 101, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Hassouneh, F.; Campos, C.; Lopez-Sejas, N.; Alonso, C.; Tarazona, R.; Solana, R.; Pera, A. Effect of age and latent CMV infection on CD8+ CD56+ T cells (NKT-like) frequency and functionality. Mech. Ageing Dev. 2016, 158, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sejas, N.; Campos, C.; Hassouneh, F.; Sanchez-Correa, B.; Tarazona, R.; Pera, A.; Solana, R. Effect of CMV and Aging on the Differential Expression of CD300a, CD161, T-bet, and Eomes on NK Cell Subsets. Front. Immunol. 2016, 7, 476. [Google Scholar] [CrossRef] [PubMed]
- Pera, A.; Campos, C.; Corona, A.; Sanchez-Correa, B.; Tarazona, R.; Larbi, A.; Solana, R. CMV latent infection improves CD8+ T response to SEB due to expansion of polyfunctional CD57+ cells in young individuals. PLoS ONE 2014, 9, e88538. [Google Scholar] [CrossRef] [PubMed]
- Solana, R.; Campos, C.; Pera, A.; Tarazona, R. Shaping of NK cell subsets by aging. Curr. Opin. Immunol. 2014, 29, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Riddell, N.E.; Griffiths, S.J.; Rivino, L.; King, D.C.; Teo, G.H.; Henson, S.M.; Cantisan, S.; Solana, R.; Kemeny, D.M.; MacAry, P.A.; et al. Multifunctional cytomegalovirus (CMV)-specific CD8(+) T cells are not restricted by telomere-related senescence in young or old adults. Immunology 2015, 144, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Misri, S.; Pandita, S.; Kumar, R.; Pandita, T.K. Telomeres, histone code, and DNA damage response. Cytogenet. Genome Res. 2008, 122, 297–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossiello, F.; Herbig, U.; Longhese, M.P.; Fumagalli, M.; di Fagagna, F.D. Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr. Opin. Genet. Dev. 2014, 26, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielak-Zmijewska, A.; Mosieniak, G.; Sikora, E. Is DNA damage indispensable for stress-induced senescence? Mech. Ageing Dev. 2018, 170, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.; Benetti, R.; Blasco, M.A. Telomerase regulation and stem cell behaviour. Curr. Opin. Cell Biol. 2006, 18, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.; Canela, A.; Vera, E.; Tejera, A.; Cotsarelis, G.; Blasco, M.A. The longest telomeres: A general signature of adult stem cell compartments. Genes Dev. 2008, 22, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.; Blasco, M.A. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010, 584, 3826–3830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar, C.; Blasco, M.A. Telomeres and telomerase as therapeutic targets to prevent and treat age-related diseases. F1000 Res. 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Blasco, M.A.; Lee, H.W.; Rizen, M.; Hanahan, D.; DePinho, R.; Greider, C.W. Mouse models for the study of telomerase. Ciba Found. Symp. 1997, 211, 160–170. [Google Scholar] [PubMed]
- Blasco, M.A. Mouse models to study the role of telomeres in cancer, aging and DNA repair. Eur. J. Cancer 2002, 38, 2222–2228. [Google Scholar] [CrossRef]
- Blasco, M.A. Telomerase beyond telomeres. Nat. Rev. Cancer 2002, 2, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Warner, J.K.; Erdmann, N.; Lansdorp, P.M.; Harrington, L.; Dick, J.E. Dissociation of telomerase activity and telomere length maintenance in primitive human hematopoietic cells. Proc. Natl. Acad. Sci. USA 2005, 102, 14398–14403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appay, V.; Dunbar, P.R.; Callan, M.; Klenerman, P.; Gillespie, G.M.; Papagno, L.; Ogg, G.S.; King, A.; Lechner, F.; Spina, C.A.; et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002, 8, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.N.; Beverley, P.C.; Salmon, M. Will telomere erosion lead to a loss of T-cell memory? Nat. Rev. Immunol. 2004, 4, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.; Blasco, M.A. Telomere-driven diseases and telomere-targeting therapies. J. Cell Biol. 2017, 216, 875–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangaonkar, A.A.; Patnaik, M.M. Short Telomere Syndromes in Clinical Practice: Bridging Bench and Bedside. Mayo Clin. Proc. 2018, 93, 904–916. [Google Scholar] [CrossRef]
- Aviv, A.; Shay, J.W. Reflections on telomere dynamics and ageing-related diseases in humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassouneh, F.; Lopez-Sejas, N.; Campos, C.; Sanchez-Correa, B.; Tarazona, R.; Solana, R.; Pera, A. Differential Effect of Cytomegalovirus Infection with Age on the Expression of CD57, CD300a, and CD161 on T-Cell Subpopulations. Front. Immunol. 2017, 8, 649. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, S.; Derhovanessian, E.; Steinhagen-Thiessen, E.; Goldeck, D.; Muller, L.; Pawelec, G. Impact of age, sex and CMV-infection on peripheral T cell phenotypes: Results from the Berlin BASE-II Study. Biogerontology 2015, 16, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Pera, A.; Campos, C.; Lopez, N.; Hassouneh, F.; Alonso, C.; Tarazona, R.; Solana, R. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas 2015, 82, 50–55. [Google Scholar] [CrossRef]
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing 2012, 9, 15. [Google Scholar] [CrossRef]
- Tarazona, R.; DelaRosa, O.; Alonso, C.; Ostos, B.; Espejo, J.; Pena, J.; Solana, R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech. Ageing Dev. 2000, 121, 77–88. [Google Scholar] [CrossRef]
- Hakim, F.T.; Flomerfelt, F.A.; Boyiadzis, M.; Gress, R.E. Aging, immunity, and cancer. Discov. Med. 2011, 11, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Larbi, A.; Derhovanessian, E. Senescence of the human immune system. J. Comp. Pathol. 2010, 142, S39–S44. [Google Scholar] [CrossRef]
- Van de Berg, P.J.; Griffiths, S.J.; Yong, S.L.; Macaulay, R.; Bemelman, F.J.; Jackson, S.; Henson, S.M.; ten Berge, I.J.; Akbar, A.N.; van Lier, R.A.; et al. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J. Immunol. 2010, 184, 3417–3423. [Google Scholar] [CrossRef]
- Dowd, J.B.; Bosch, J.A.; Steptoe, A.; Blackburn, E.H.; Lin, J.; Rees-Clayton, E.; Aiello, A.E. Cytomegalovirus is associated with reduced telomerase activity in the Whitehall II cohort. Exp. Gerontol. 2013, 48, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiello, A.E.; Jayabalasingham, B.; Simanek, A.M.; ez-Roux, A.; Feinstein, L.; Meier, H.C.S.; Needham, B.L.; Dowd, J.B. The impact of pathogen burden on leukocyte telomere length in the Multi-Ethnic Study of Atherosclerosis. Epidemiol. Infect. 2017, 145, 3076–3084. [Google Scholar] [CrossRef]
- Dowd, J.B.; Bosch, J.A.; Steptoe, A.; Jayabalasingham, B.; Lin, J.; Yolken, R.; Aiello, A.E. Persistent Herpesvirus Infections and Telomere Attrition Over 3 Years in the Whitehall II Cohort. J. Infect. Dis. 2017, 216, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lustig, A.; Liu, H.B.; Metter, E.J.; Macaulay, R.; Bemelman, F.J.; Jackson, S.; Henson, S.M.; ten Berge, I.J.; Akbar, A.N.; van Lier, R.A. Telomere Shortening, Inflammatory Cytokines, and Anti-Cytomegalovirus Antibody Follow Distinct Age-Associated Trajectories in Humans. Front. Immunol. 2017, 8, 1027. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.P.; Effros, R.B. T cell replicative senescence in human aging. Curr. Pharm. Des. 2013, 19, 1680–1698. [Google Scholar] [PubMed]
- Collerton, J.; Martin-Ruiz, C.; Davies, K.; Hilkens, C.M.; Isaacs, J.; Kolenda, C.; Parker, C.; Dunn, M.; Catt, M.; Jagger, C.; et al. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: Cross-sectional findings from the Newcastle 85+ Study. Mech. Ageing Dev. 2012, 133, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.B. Telomere/telomerase dynamics within the human immune system: Effect of chronic infection and stress. Exp. Gerontol. 2011, 46, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, J.M.; Vukmanovic-Stejic, M.; Dunne, P.J.; Birch, K.E.; Cook, J.E.; Jackson, S.E.; Salmon, M.; Rustin, M.H.; Akbar, A.N. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J. Immunol. 2005, 175, 8218–8225. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Immunosenenescence: Role of cytomegalovirus. Exp. Gerontol. 2014, 54, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G. Human T cell aging and the impact of persistent viral infections. Front. Immunol. 2013, 4, 271. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Zhou, X.; Talbot, H.K.; Soethout, E.; Bleackley, R.C.; Granville, D.J.; Pawelec, G. The unmet need in the elderly: How immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012, 30, 2060–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawelec, G.; Derhovanessian, E. Role of CMV in immune senescence. Virus Res. 2011, 157, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Thomasini, R.L.; Pereira, D.S.; Pereira, F.S.M.; Mateo, E.C.; Mota, T.N.; Guimarães, G.G.; Pereira, L.S.M.; Lima, C.X.; Teixeira, M.M.; Teixeira, A.L. Junior. Aged-associated cytomegalovirus and Epstein-Barr virus reactivation and cytomegalovirus relationship with the frailty syndrome in older women. PLoS ONE 2017, 12, e0180841. [Google Scholar] [CrossRef] [PubMed]
- Cao-Dinh, H.; Bautmans, I.; Beyer, I.; Mets, T.; Onyema, O.O.; Forti, L.N.; Renmans, W.; Vander Meeren, S.; Jochmans, K.; Vermeiren, S.; et al. Association between Immunosenescence Phenotypes and pre-frailty in Older Subjects: Does Cytomegalovirus Play a Role? J. Gerontol. A Biol. Sci. Med. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Elwenspoek, M.M.C.; Sias, K.; Hengesch, X.; Schaan, V.K.; Leenen, F.A.D.; Adams, P.; Mériaux, S.B.; Schmitz, S.; Bonnemberger, F.; Ewen, A.; et al. T Cell Immunosenescence after Early Life Adversity: Association with Cytomegalovirus Infection. Front. Immunol. 2017, 8, 1263. [Google Scholar] [CrossRef] [PubMed]
- SBakaysa, L.; Mucci, L.A.; Slagboom, P.E.; Boomsma, D.I.; McClearn, G.E.; Johansson, B.; Pedersen, N.L. Telomere length predicts survival independent of genetic influences. Aging Cell 2007, 6, 769–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Alvira, J.M.; Fuster, V.; Dorado, B.; Soberón, N.; Flores, I.; Gallardo, M.; Pocock, S.; Blasco, M.A.; Andrés, V. Short Telomere Load, Telomere Length, and Subclinical Atherosclerosis: The PESA Study. J. Am. Coll. Cardiol. 2016, 67, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.L.; Rehkopf, D.; Adler, N.; Gregorich, S.; Lin, J.; Blackburn, E.H.; Epel, E.S. Leukocyte telomere length and mortality in the National Health and Nutrition Examination Survey, 1999–2002. Epidemiology 2015, 26, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sun, J.; Wang, S.; Milush, J.M.; Baker, C.A.R.; Coccia, M.; Effros, R.B.; Puterman, E.; Blackburn, E.; Prather, A.A.; et al. In vitro proinflammatory gene expression predicts in vivo telomere shortening: A preliminary study. Psychoneuroendocrinology 2018, 96, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Belcher, M.; Van Der Harst, P. Healthy aging and disease: Role for telomere biology? Clin. Sci. 2011, 120, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Harari, Y.; Kupiec, M. Do long telomeres affect cellular fitness? Curr. Genet. 2018, 64, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Opresko, P.L.; Shay, J.W. Telomere-associated aging disorders. Ageing Res. Rev. 2017, 33, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Astuti, Y.; Wardhana, A.; Watkins, J.; Wulaningsih, W. Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Environ. Res. 2017, 158, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kim, H.; Baik, I. Associations of alcohol consumption and alcohol flush reaction with leukocyte telomere length in Korean adults. Nutr. Res. Pract. 2017, 11, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Hwang, S.S.; Choi, J.R.; Lee, S.T.; Kim, J.; Hwang, I.S.; Kim, H.W.; Kim, C.H.; Kim, S.J. Telomere length in alcohol dependence: A role for impulsive choice and childhood maltreatment. Psychoneuroendocrinology 2017, 83, 72–78. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, T.; Nawrot, T.; Bekaert, S.; De Buyzere, M.L.; Rietzschel, E.R.; Andrés, V. Telomere Length as Cardiovascular Aging Biomarker: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Pusceddu, I.; Marz, W.; Herrmann, W. Telomere biology and age-related diseases. Clin. Chem. Lab. Med. 2018, 56, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Fougere, B.; Boulanger, E.; Nourhashemi, F.; Guyonnet, S.; Cesari, M. Chronic Inflammation: Accelerator of Biological Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.K.; Wang, C.Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Mons, U.; Muezzinler, A.; Schottker, B.; Dieffenbach, A.K.; Butterbach, K.; Schick, M.; Peasey, A.; De Vivo, I.; Trichopoulou, A.; Boffetta, P.; et al. Leukocyte Telomere Length and All-Cause, Cardiovascular Disease, and Cancer Mortality: Results from Individual-Participant-Data Meta-Analysis of 2 Large Prospective Cohort Studies. Am. J. Epidemiol. 2017, 185, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Bersani, F.S.; Lindqvist, D.; Mellon, S.H.; Epel, E.S.; Yehuda, R.; Flory, J.; Henn-Hasse, C.; Bierer, L.M.; Makotkine, I.; Abu-Amara, D.; et al. Association of dimensional psychological health measures with telomere length in male war veterans. J. Affect. Disord. 2016, 190, 537–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darrow, S.M.; Verhoeven, J.E.; Revesz, D.; Lindqvist, D.; Penninx, B.W.; Delucchi, K.L.; Wolkowitz, O.M.; Mathews, C.A. The Association between Psychiatric Disorders and Telomere Length: A Meta-Analysis Involving 14,827 Persons. Psychosom. Med. 2016, 78, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Epel, E.S.; Mellon, S.H.; Penninx, B.W.; Révész, D.; Verhoeven, J.E.; Reus, V.I.; Lin, J.; Mahan, L.; Hough, C.M.; et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci. Biobehav. Rev. 2015, 55, 333–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouilette, S.W.; Moore, J.S.; McMahon, A.D.; Thompson, J.R.; Ford, I.; Shepherd, J.; Packard, C.J.; Samani, N.J. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: A nested case-control study. Lancet 2007, 369, 107–114. [Google Scholar] [CrossRef]
- Farzaneh-Far, R.; Cawthon, R.M.; Na, B.; Browner, W.S.; Schiller, N.B.; Whooley, M.A. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: Data from the Heart and Soul Study. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Spyridopoulos, I.; von Zglinicki, T. Telomere length predicts cardiovascular disease. BMJ 2014, 349, g4373. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, J.; Matsuguchi, T.; Blackburn, E.; Yeh, F.; Best, L.G.; Devereux, R.B.; Lee, E.T.; Howard, B.V.; Roman, M.J.; et al. Short leukocyte telomere length predicts incidence and progression of carotid atherosclerosis in American Indians: The Strong Heart Family Study. Aging 2014, 6, 414–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goglin, S.E.; Farzaneh-Far, R.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Whooley, M.A. Change in Leukocyte Telomere Length Predicts Mortality in Patients with Stable Coronary Heart Disease from the Heart and Soul Study. PLoS ONE 2016, 11, e0160748. [Google Scholar]
- Hoffmann, J.; Shmeleva, E.V.; Boag, S.E.; Fiser, K.; Bagnall, A.; Murali, S.; Dimmick, I.; Pircher, H.; Martin-Ruiz, C.; Egred, M.; et al. Myocardial ischemia and reperfusion leads to transient CD8 immune deficiency and accelerated immunosenescence in CMV-seropositive patients. Circ. Res. 2015, 116, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yeh, F.; Lin, J.; Matsuguchi, T.; Blackburn, E.; Lee, E.T.; Howard, B.V.; Zhao, J. Short leukocyte telomere length is associated with obesity in American Indians: The Strong Heart Family study. Aging 2014, 6, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, Y.; Uppal, K.; Tran, V.T.; Yu, T.; Lin, J.; Matsuguchi, T.; Blackburn, E.; Jones, D.; Lee, E.T.; et al. Metabolic profiles of biological aging in American Indians: The Strong Heart Family Study. Aging 2014, 6, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Testa, R.; Olivieri, F.; Sirolla, C.; Spazzafumo, L.; Rippo, M.R.; Marra, M.; Bonfigli, A.R.; Ceriello, A.; Antonicelli, R.; Franceschi, C.; et al. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabet. Med. 2011, 28, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.L.; Yiamunaa, M.; Liu, S.; Liu, J.J.; Lim, S.C. Short Leukocyte Telomere Length Predicts Albuminuria Progression in Individuals with Type 2 Diabetes. Kidney Int. Rep. 2018, 3, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.C.C.C.; Santos, R.R.C.D.; Fernandes, L.F.A.; Neves, F.A.R.; Coelho, M.S.; Amato, A.A. Leukocyte telomere length correlates with glucose control in adults with recently diagnosed type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 135, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Sethi, I.; Bhat, G.R.; Singh, V.; Kumar, R.; Bhanwer, A.J.; Bamezai, R.N.; Sharma, S.; Rai, E. Role of telomeres and associated maintenance genes in Type 2 Diabetes Mellitus: A review. Diabetes Res. Clin. Pract. 2016, 122, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Bonfigli, A.R.; Spazzafumo, L.; Prattichizzo, F.; Bonafè, M.; Mensà, E.; Micolucci, L.; Giuliani, A.; Fabbietti, P.; Testa, R.; Boemi, M.; et al. Leukocyte telomere length and mortality risk in patients with type 2 diabetes. Oncotarget 2016, 7, 50835–50844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spigoni, V.; Aldigeri, R.; Picconi, A.; Derlindati, E.; Franzini, L.; Haddoub, S.; Prampolini, G.; Vigna, G.B.; Zavaroni, I.; Bonadonna, R.C.; et al. Telomere length is independently associated with subclinical atherosclerosis in subjects with type 2 diabetes: A cross-sectional study. Acta Diabetol. 2016, 53, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Takubo, K.; Aida, J.; Araki, A.; Ito, H. Telomere attrition and diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16, 66–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, R.L.; Yiamunaa, M.; Liu, S.; Liu, J.J.; Chan, S.M.; Moh, M.C.; Ang, K.; Tang, W.E.; Sum, C.F.; Subramaniam, T.; et al. Ethnic disparities in relationship of obesity indices and telomere length in Asians with type 2 diabetes. J. Diabetes 2018. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.E.; Revesz, D.; Epel, E.S.; Lin, J.; Wolkowitz, O.M.; Penninx, B.W. Major depressive disorder and accelerated cellular aging: Results from a large psychiatric cohort study. Mol. Psychiatry 2014, 19, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.E.; Revesz, D.; van Oppen, P.; Epel, E.S.; Wolkowitz, O.M.; Penninx, B.W. Anxiety disorders and accelerated cellular ageing. Br. J. Psychiatry 2015, 206, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolkowitz, O.M.; Jeste, D.V.; Martin, A.S.; Lin, J.; Daly, R.E.; Reuter, C.; Kraemer, H. Leukocyte telomere length: Effects of schizophrenia, age, and gender. J. Psychiatr. Res. 2017, 85, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolkowitz, O.M.; Mellon, S.H.; Epel, E.S.; Lin, J.; Dhabhar, F.S.; Su, Y.; Reus, V.I.; Rosser, R.; Burke, H.M.; Kupferman, E.; et al. Leukocyte telomere length in major depression: Correlations with chronicity, inflammation and oxidative stress—Preliminary findings. PLoS ONE 2011, 6, e17837. [Google Scholar] [CrossRef] [PubMed]
- Ridout, K.K.; Ridout, S.J.; Price, L.H.; Sen, S.; Tyrka, A.R. Depression and telomere length: A meta-analysis. J. Affect. Disord. 2016, 191, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wium-Andersen, M.K.; Orsted, D.D.; Rode, L.; Bojesen, S.E.; Nordestgaard, B.G. Telomere length and depression: Prospective cohort study and Mendelian randomisation study in 67 306 individuals. Br. J. Psychiatry 2017, 210, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.C.; Bui, E.; Hoeppner, S.S.; Kovachy, B.; Prescott, J.; Mischoulon, D.; Walton, Z.E.; Dong, M.; Nadal, M.F.; Worthington, J.J.; et al. Prospective association between major depressive disorder and leukocyte telomere length over two years. Psychoneuroendocrinology 2018, 90, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.L.; Mezuk, B.; Bareis, N.; Lin, J.; Blackburn, E.H.; Epel, E.S. Depression, anxiety and telomere length in young adults: Evidence from the National Health and Nutrition Examination Survey. Mol. Psychiatry 2015, 20, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Hough, C.M.; Bersani, F.S.; Mellon, S.H.; Epel, E.S.; Reus, V.I.; Lindqvist, D.; Lin, J.; Mahan, L.; Rosser, R.; Burke, H.; et al. Leukocyte telomere length predicts SSRI response in major depressive disorder: A preliminary report. Mol. Neuropsychiatry 2016, 2, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Revesz, D.; Verhoeven, J.E.; Milaneschi, Y.; Penninx, B.W. Depressive and anxiety disorders and short leukocyte telomere length: Mediating effects of metabolic stress and lifestyle factors. Psychol. Med. 2016, 46, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Blomstrom, A.; Gardner, R.M.; Dalman, C.; Yolken, R.H.; Karlsson, H. Influence of maternal infections on neonatal acute phase proteins and their interaction in the development of non-affective psychosis. Transl. Psychiatry 2015, 5, e502. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Weber, N.S.; Fisher, J.A.; Yolken, R.H.; Cowan, D.N.; Larsen, R.A.; Niebuhr, D.W. Association between antibodies to multiple infectious and food antigens and new onset schizophrenia among US military personnel. Schizophr. Res. 2013, 151, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohagheghi, M.; Eftekharian, M.M.; Taheri, M.; Alikhani, M.Y. Determining the IgM and IgG antibodies titer against HSV1, HSV2 and CMV in the serum of schizophrenia patients. Hum. Antibodies 2018, 26, 87–93. [Google Scholar] [CrossRef] [PubMed]
- De Witte, L.D.; van Mierlo, H.C.; Litjens, M.; Klein, H.C.; Bahn, S.; Osterhaus, A.D. The association between antibodies to neurotropic pathogens and schizophrenia: A case-control study. NPJ Schizophr. 2015, 1, 15041. [Google Scholar] [CrossRef] [PubMed]
- Bolu, A.; Oznur, T.; Tok, D.; Balikci, A.; Sener, K.; Celik, C.; Gulsun, M. Seropositivity of neurotropic infectious agents in first-episode schizophrenia patients and the relationship with positive and negative symptoms. Psychiatr. Danub. 2016, 28, 132–138. [Google Scholar] [PubMed]
- Houenou, J.; d’Albis, M.A.; Daban, C.; Hamdani, N.; Delavest, M.; Lepine, J.P.; Vederine, F.E.; Carde, S.; Lajnef, M.; Cabon, C.; et al. Cytomegalovirus seropositivity and serointensity are associated with hippocampal volume and verbal memory in schizophrenia and bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, F.; Wilcox, H.C.; Adamos, M.; Katsafanas, E.; Khushalani, S.; Origoni, A.; Savage, C.; Schweinfurth, L.; Stallings, C.; Sweeney, K.; et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J. Psychiatr. Res. 2017, 87, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Origoni, A.; Schweinfurth, L.A.B.; Stallings, C.; Savage, C.L.G.; Sweeney, K.; Katsafanas, E.; Wilcox, H.C.; Khushalani, S.; Yolken, R. Clinical and Serological Predictors of Suicide in Schizophrenia and Major Mood Disorders. J. Nerv. Ment. Dis. 2018, 206, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Okusaga, O.O. Accelerated aging in schizophrenia patients: The potential role of oxidative stress. Aging Dis. 2014, 5, 256–262. [Google Scholar] [PubMed]
- Horvath, S.; Mirnics, K. Immune system disturbances in schizophrenia. Biol. Psychiatry 2014, 75, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Amorim, D.; Rivera-Baltanas, T.; Spuch, C.; Caruncho, H.J.; Gonzalez-Fernandez, A.; Olivares, J.M.; Agis-Balboa, R.C. Cytokines dysregulation in schizophrenia: A systematic review of psychoneuroimmune relationship. Schizophr. Res. 2017, 197, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Na, K.S.; Jung, H.Y.; Kim, Y.K. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, S.; Trabace, L. Inflammation, Stress Response, and Redox Dysregulation Biomarkers: Clinical Outcomes and Pharmacological Implications for Psychosis. Front Psychiatry 2017, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Fraguas, D.; az-Caneja, C.M.; Rodriguez-Quiroga, A.; Arango, C. Oxidative Stress and Inflammation in Early Onset First Episode Psychosis: A Systematic Review and Meta-Analysis. Int. J. Neuropsychopharmacol. 2017, 20, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Polho, G.B.; De-Paula, V.J.; Cardillo, G.; dos Santos, B.; Kerr, D.S. Leukocyte telomere length in patients with schizophrenia: A meta-analysis. Schizophr. Res. 2015, 165, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Kota, L.N.; Li, Z.; Yao, Y.; Tang, J.; Mao, C.; Jain, S.; Xu, Y.; Xu, Q. Accelerated leukocyte telomere erosion in schizophrenia: Evidence from the present study and a meta-analysis. J. Psychiatr. Res. 2016, 79, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Ye, N.; Hu, H.; Shen, Y.; Xu, Q. Variants in TERT influencing telomere length are associated with paranoid schizophrenia risk. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Galletly, C.; Dhillon, V.S.; Liu, D.; Balzan, R.P.; Hahn, L.A.; Fenech, M.F. Shorter telomere length in people with schizophrenia: A preliminary study from Australia. Schizophr. Res. 2017, 190, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Vaez-Azizi, L.M.; Ruby, E.; Dracxler, R.; Rothman, K.; Perrin, M.; Walsh-Messinger, J.; Antonius, D.; Goetz, R.R.; Goetz, D.M.; Keefe, D.L.; et al. Telomere length variability is related to symptoms and cognition in schizophrenia. Schizophr. Res. 2015, 164, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Chowdari, K.; Fathi, W.; Elassy, M.; Ibrahim, I.; Wood, J.; Bamne, M.; Tobar, S.; Yassin, A.; Salah, H.; et al. Does telomere length mediate associations between inbreeding and increased risk for bipolar I disorder and schizophrenia? Psychiatry Res. 2011, 188, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Nieratschker, V.; Lahtinen, J.; Meier, S.; Strohmaier, J.; Frank, J.; Heinrich, A.; Breuer, R.; Witt, S.H.; Nöthen, M.M.; Rietschel, M.; et al. Longer telomere length in pattiens with schizophrenia. Schizophr. Res. 2013, 149, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.K.; Rizzo, L.B.; Xavier, G.; Tempaku, P.F.; Ota, V.K.; Santoro, M.L.; Spíndola, L.M.; Moretti, P.S.; Mazzotti, D.R.; Gadelha, A.; et al. Leukocyte telomere length variation in different stages of schizophrenia. J. Psychiatr. Res. 2018, 96, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.K.; Rizzo, L.B.; Xavier, G.; Tempaku, P.F.; Zeni-Graiff, M.; Santoro, M.L.; Mazzotti, D.R.; Zugman, A.; Pan, P.; Noto, C.; et al. Shorter leukocyte telomere length in patients at ultra high risk for psychosis. Eur. Neuropsychopharmacol. 2017, 27, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Malaspina, D.; Walsh-Messinger, J.; Antonius, D.; Dracxler, R.; Rothman, K.; Puthota, J.; Gilman, C.; Feuerstein, J.L.; Keefe, D.; Goetz, D.; et al. Parental age effects on odor sensitivity in healthy subjects and schizophrenia patients. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.E.; Hunt, S.C.; Stone, R.C.; Horvath, K.; Herbig, U.; Ranciaro, A.; Hirbo, J.; Beggs, W.; Reiner, A.P.; Wilson, J.G.; et al. Shorter telomere length in Europeans than in Africans due to polygenetic adaptation. Hum. Mol. Genet. 2016, 25, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Czepielewski, L.S.; Massuda, R.; Panizzutti, B.; Grun, L.K.; Barbe-Tuana, F.M.; Teixeira, A.L.; Barch, D.M.; Gama, C.S. Telomere Length and CCL11 Levels are Associated with Gray Matter Volume and Episodic Memory Performance in Schizophrenia: Evidence of Pathological Accelerated Aging. Schizophr. Bull. 2018, 44, 158–167. [Google Scholar] [CrossRef] [PubMed]
- King, K.S.; Kozlitina, J.; Rosenberg, R.N.; Peshock, R.M.; McColl, R.W.; Garcia, C.K. Effect of leukocyte telomere length on total and regional brain volumes in a large population-based cohort. JAMA Neurol. 2014, 71, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Prinzi, G.; Proietti, S.; Lamonaca, P.; Frustaci, A.; Boccia, S.; Amore, R.; Lorenzi, M.; Onder, G.; Marzetti, E.; et al. Shorter telomere length in schizophrenia: Evidence from a real-world population and meta-analysis of most recent literature. Schizophr. Res. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Porton, B.; Delisi, L.E.; Bertisch, H.C.; Ji, F.; Gordon, D.; Li, P.; Benedict, M.M.; Greenberg, W.M.; Kao, H.T. Telomerase levels in schizophrenia: A preliminary study. Schizophr. Res. 2008, 106, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alder, J.K.; Parry, E.M.; Yegnasubramanian, S.; Wagner, C.L.; Lieblich, L.M.; Auerbach, R.; Auerbach, A.D.; Wheelan, S.J.; Armanios, M. Telomere phenotypes in females with heterozygous mutations in the dyskeratosis congenita 1 (DKC1) gene. Hum. Mutat. 2013, 34, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.E.; Armanios, M. The short and long telomere syndromes: Paired paradigms for molecular medicine. Curr. Opin. Genet. Dev. 2015, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, F.; Saijonmaa, O. Telomere length and cardiovascular aging. Ann. Med. 2012, 44 (Suppl. 1), S138–S142. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.; Perrin, M.; Vaez-Azizi, L.M.; Ruby, E.; Goetz, R.R.; Dracxler, R.; Walsh-Messinger, J.; Keefe, D.L.; Buckley, P.F.; Szeszko, P.R.; et al. Telomere length and early trauma in schizophrenia. Schizophr. Res. 2018, 199, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Balzan, R.P.; Dhillon, V.S.; Liu, D.; Hahn, L.; Fenech, M.F.; Galletly, C. Shorter telomere length in people with schizophrenia who live alone? Schizophr. Res. 2018, 199, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Cropley, V.L.; Klauser, P.; Lenroot, R.K.; Bruggemann, J.; Sundram, S.; Bousman, C.; Pereira, A.; Di Biase, M.A.; Weickert, T.W.; Weickert, C.S.; et al. Accelerated Gray and White Matter Deterioration with Age in Schizophrenia. Am. J. Psychiatry 2017, 174, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Van Mierlo, H.C.; Wichers, C.G.K.; He, Y.; Sneeboer, M.A.M.; Radstake, T.R.D.J.; Kahn, R.S.; Broen, J.C.A.; de Witte, L.D. Telomere quantification in frontal and temporal brain tissue of patients with schizophrenia. J. Psychiatr. Res. 2017, 95, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Schnack, H.G.; van Haren, N.E.; Nieuwenhuis, M.; Pol, H.E.H.; Cahn, W.; Kahn, R.S. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am. J. Psychiatry 2016, 173, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Drexhage, R.C.; Weigelt, K.; van Beveren, N.; Cohen, D.; Versnel, M.A.; Nolen, W.A.; Drexhage, H.A. Immune and neuroimmune alterations in mood disorders and schizophrenia. Int. Rev. Neurobiol. 2011, 101, 169–201. [Google Scholar] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solana, C.; Pereira, D.; Tarazona, R. Early Senescence and Leukocyte Telomere Shortening in SCHIZOPHRENIA: A Role for Cytomegalovirus Infection? Brain Sci. 2018, 8, 188. https://doi.org/10.3390/brainsci8100188
Solana C, Pereira D, Tarazona R. Early Senescence and Leukocyte Telomere Shortening in SCHIZOPHRENIA: A Role for Cytomegalovirus Infection? Brain Sciences. 2018; 8(10):188. https://doi.org/10.3390/brainsci8100188
Chicago/Turabian StyleSolana, Corona, Diana Pereira, and Raquel Tarazona. 2018. "Early Senescence and Leukocyte Telomere Shortening in SCHIZOPHRENIA: A Role for Cytomegalovirus Infection?" Brain Sciences 8, no. 10: 188. https://doi.org/10.3390/brainsci8100188