Somatosensory Event-Related Potential as an Electrophysiological Correlate of Endogenous Spatial Tactile Attention: Prospects for Electrotactile Brain-Computer Interface for Sensory Training
Abstract
:1. Introduction
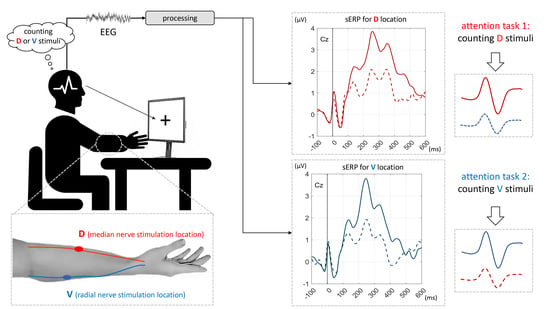
2. Materials and Methods
2.1. Participants
2.2. Apparatus and Instrumentation
2.3. Protocol
- Attending stimuli delivered to D location, while D location was being stimulated: ADSD—attending D, stimulated D,
- Attending stimuli delivered to D location, while V location was being stimulated: ADSV—attending D, stimulated V,
- Attending stimuli delivered to V location, while V location was being stimulated: AVSV—attending V, stimulated V, and
- Attending stimuli delivered to V location, while D location was being stimulated: AVSD—attending V, stimulated D.
2.4. ERP Processing
2.5. Statistical Analysis
3. Results
- Positive peak marked as P100, at latencies ranging from 75 to 135 ms over different EEG channels,
- Negative peak marked as N140, at latencies ranging from 115 to 165 ms over different EEG channels,
- First positive local maximum within a larger window between 200 and 400 ms, termed P3a, and
- Second positive local maximum within a larger window between 200 and 400 ms, termed P3b.
4. Discussion
- (1)
- The stimulation of the mixed nerve branches instead of exclusively sensory nerve branches commonly used in basic ERP studies exploring tactile attention.
- (2)
- The electrotactile attention task involving two stimulation hotspots with equal (50%) probability of stimulus occurrence, unlike commonly used oddball paradigm with rare and frequent stimulus.
4.1. Somatosensory ERP Morphology Associated with Mixed Nerve Stimulation
4.2. Effects of Endogenous Spatial Electrotactile Attention on Somatosensory ERP Components
4.2.1. P100 Component
4.2.2. N140 Component
4.2.3. P3a and P3b Component
4.3. Prospects for Tactile BCI Applications
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomez-Ramirez, M.; Hysaj, K.; Niebur, E. Neural Mechanisms of Selective Attention in the Somatosensory System. J. Neurophysiol. 2016, 116, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Sambo, C.F.; Forster, B. Sustained Spatial Attention in Touch: Modality-Specific and Multimodal Mechanisms. Sci. World J. 2011, 11, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, J. The Effect of Tactile Training on Sustained Attention in Young Adults. Brain Sci. 2020, 10, 695–710. [Google Scholar] [CrossRef] [PubMed]
- McCracken, H.S.; Murphy, B.A.; Ambalavanar, U.; Glazebrook, C.M.; Yielder, P.C. Source Localization of Somatosensory Neural Generators in Adults with Attention-Deficit/Hyperactivity Disorder. Brain Sci. 2023, 13, 370. [Google Scholar] [CrossRef]
- Cascio, C.J.; Gu, C.; Schauder, K.B.; Key, A.P.; Yoder, P. Somatosensory Event-Related Potentials and Association with Tactile Behavioral Responsiveness Patterns in Children with ASD. Brain Topogr. 2015, 28, 895–903. [Google Scholar] [CrossRef]
- He, J.L.; Wodka, E.; Tommerdahl, M.; Edden, R.A.E.; Mikkelsen, M.; Mostofsky, S.H.; Puts, N.A.J. Disorder-Specific Alterations of Tactile Sensitivity in Neurodevelopmental Disorders. Commun. Biol. 2021, 4, 97. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Yu, Y.; Li, H.; Liu, Y.; Yu, Y.; Yu, J.; Yang, J.; Takahashi, S.; Ejima, Y.; et al. Tactile Angle Discriminability Improvement: Contributions of Working Memory Training and Continuous Attended Sensory Input. J. Neurophysiol. 2022, 127, 1389–1406. [Google Scholar] [CrossRef]
- Mallory, C.; Keehn, B. Implications of Sensory Processing and Attentional Differences Associated With Autism in Academic Settings: An Integrative Review. Front. Psychiatry 2021, 12, 695825. [Google Scholar] [CrossRef]
- Barutchu, A.; Toohey, S.; Shivdasani, M.N.; Fifer, J.M.; Crewther, S.G.; Grayden, D.B.; Paolini, A.G. Multisensory Perception and Attention in School-Age Children. J. Exp. Child. Psychol. 2019, 180, 141–155. [Google Scholar] [CrossRef]
- Debowska, W.; Wolak, T.; Nowicka, A.; Kozak, A.; Szwed, M.; Kossut, M. Functional and Structural Neuroplasticity Induced by Short-Term Tactile Training Based on Braille Reading. Front. Neurosci. 2016, 10, 460. [Google Scholar] [CrossRef]
- Carey, L.M.; Matyas, T.A.; Oke, L.E. Sensory Loss in Stroke Patients: Effective Training of Tactile and Proprioceptive Discrimination. Arch. Phys. Med. Rehabil. 1993, 74, 602–611. [Google Scholar] [CrossRef]
- Dechaumont-Palacin, S.; Marque, P.; De Boissezon, X.; Castel-Lacanal, E.; Carel, C.; Berry, I.; Pastor, J.; Albucher, J.F.; Chollet, F.; Loubinoux, I. Neural Correlates of Proprioceptive Integration in the Contralesional Hemisphere of Very Impaired Patients Shortly after a Subcortical Stroke: An FMRI Study. Neurorehabil. Neural Repair 2008, 22, 154–165. [Google Scholar] [CrossRef]
- Aman, J.E.; Elangovan, N.; Yeh, I.L.; Konczak, J. The Effectiveness of Proprioceptive Training for Improving Motor Function: A Systematic Review. Front. Hum. Neurosci. 2015, 8, 1075. [Google Scholar] [CrossRef]
- Josiassen, R.C.; Shagass, C.; Roemer, R.A.; Slepner, S.; Czartorysky, B. Early Cognitive Components of Somatosensory Event-Related Potentials. Int. J. Psychophysiol. 1990, 9, 139–149. [Google Scholar] [CrossRef]
- Haider, A.; Fazel-Rezai, R. Application of P300 Event-Related Potential in Brain-Computer Interface. Event-Relat. Potentials Evoked Potentials 2017, 1, 19–36. [Google Scholar] [CrossRef]
- Ramot, M.; Martin, A. Closed-Loop Neuromodulation for Studying Spontaneous Activity and Causality. Trends Cogn. Sci. 2022, 26, 290–299. [Google Scholar] [CrossRef]
- Savić, A.M.; Novičić, M.; Ðorđević, O.; Konstantinović, L.; Miler-Jerković, V. Novel Electrotactile Brain-Computer Interface with Somatosensory Event-Related Potential Based Control. Front. Hum. Neurosci. 2023, 17, 1096814. [Google Scholar] [CrossRef]
- Ward, N.S. Mechanisms Underlying Recovery of Motor Function after Stroke. Postgrad. Med. J. 2005, 81, 510–514. [Google Scholar] [CrossRef]
- Nicolas-Alonso, L.F.; Gomez-Gil, J. Brain Computer Interfaces, a Review. Sensors 2012, 12, 1211–1279. [Google Scholar] [CrossRef]
- Ramadan, R.A.; Vasilakos, A.V. Brain Computer Interface: Control Signals Review. Neurocomputing 2017, 223, 26–44. [Google Scholar] [CrossRef]
- Woodman, G.F. A Brief Introduction to the Use of Event-Related Potentials in Studies of Perception and Attention. Atten. Percept. Psychophys. 2010, 72, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- Michie, P.T.; Bearparic, H.M.; Crawford, J.M.; Glue, L.C. The Effects of Spatial Selective Attention on the Somatosensory Event-Related Potential. Psychophysiology 1987, 24, 449–463. [Google Scholar] [CrossRef]
- Kida, T.; Nishihira, Y.; Wasaka, T.; Nakata, H.; Sakamoto, M. Passive Enhancement of the Somatosensory P100 and N140 in an Active Attention Task Using Deviant Alone Condition. Clin. Neurophysiol. 2004, 115, 871–879. [Google Scholar] [CrossRef]
- Kida, T.; Wasaka, T.; Nakata, H.; Akatsuka, K.; Kakigi, R. Active Attention Modulates Passive Attention-Related Neural Responses to Sudden Somatosensory Input against a Silent Background. Exp. Brain Res. 2006, 175, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Petrusic, I.; Jovanovic, V.; Kovic, V.; Savic, A. Characteristics of N400 Component Elicited in Patients Who Have Migraine with Aura. J. Headache Pain 2021, 22, 157. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, J.E.; Tomberg, C. Mapping Early Somatosensory Evoked Potentials in Selective Attention: Critical Evaluation of Control Conditions Used for Titrating by Difference the Cognitive P30, P40, P100 and N140. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1989, 74, 321–346. [Google Scholar] [CrossRef]
- Adams, M.S.; Niechwiej-Szwedo, E.; McIlroy, W.E.; Staines, W.R. A History of Concussion Affects Relevancy-Based Modulation of Cortical Responses to Tactile Stimuli. Front. Integr. Neurosci. 2020, 14, 33. [Google Scholar] [CrossRef]
- Schubert, R.; Ritter, P.; Wüstenberg, T.; Preuschhof, C.; Curio, G.; Sommer, W.; Villringer, A. Spatial Attention Related SEP Amplitude Modulations Covary with BOLD Signal in S1—A Simultaneous EEG—FMRI Study. Cereb. Cortex 2008, 18, 2686–2700. [Google Scholar] [CrossRef]
- Kida, T.; Nishihira, Y.; Hatta, A.; Wasaka, T.; Tazoe, T.; Sakajiri, Y.; Nakata, H.; Kaneda, T.; Kuroiwa, K.; Akiyama, S.; et al. Resource Allocation and Somatosensory P300 Amplitude during Dual Task: Effects of Tracking Speed and Predictability of Tracking Direction. Clin. Neurophysiol. 2004, 115, 2616–2628. [Google Scholar] [CrossRef]
- Nakata, H.; Inui, K.; Wasaka, T.; Nishihira, Y.; Kakigi, R. Mechanisms of Differences in Gating Effects on Short-and Long-Latency Somatosensory Evoked Potentials Relating to Movement. Brain Topogr. 2003, 15, 211–222. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Robertson, D. Differential Enhancement of Early and Late Components of the Cerebral Somatosensory Evoked Potentials during Forced-Paced Cognitive Tasks in Man. J. Physiol. 1977, 271, 761–769. [Google Scholar] [CrossRef]
- Luck, S.J.; Heinze, H.J.; Mangun, G.R.; Hillyard, S.A. Visual Event-Related Potentials Index Focused Attention within Bilateral Stimulus Arrays. II. Functional Dissociation of P1 and N1 Components. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 528–542. [Google Scholar] [CrossRef]
- Allison, T.; Mccarthy, G.; Wood, C.C. The Relationship between Human Long-Latency Somatosensory Evoked Potentials Recorded from the Cortical Surface and from the Scalp. Electroencephalogr. Clin. Neurophysiol. 1992, 84, 301–314. [Google Scholar] [CrossRef]
- Kida, T.; Nishihira, Y.; Wasaka, T.; Nakata, H.; Sakamoto, M. Differential Modulation of Temporal and Frontal Components of the Somatosensory N140 and the Effect of Interstimulus Interval in a Selective Attention Task. Cogn. Brain Res. 2004, 19, 33–39. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.; Lukaszewicz, A.-C.; Mauguiere, F. Somatosensory Responses during Selective Spatial Attention: The N120-to-N140 Transition. Psychophysiology 1995, 32, 526–537. [Google Scholar] [CrossRef]
- Franz, M.; Nickel, M.M.; Ritter, A.; Miltner, W.H.R.; Weiss, T. Somatosensory Spatial Attention Modulates Amplitudes, Latencies, and Latency Jitter of Laser-Evoked Brain Potentials. J. Neurophysiol. 2015, 113, 2760–2768. [Google Scholar] [CrossRef]
- Polich, J.; Criado, J.R. Neuropsychology and Neuropharmacology of P3a and P3b. Int. J. Psychophysiol. 2006, 60, 172–185. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An Integrative Theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Gaeta, H.; Friedman, D.; Hunt, G. Stimulus Characteristics and Task Category Dissociate the Anterior and Posterior Aspects of the Novelty P3. Psychophysiology 2003, 40, 198–208. [Google Scholar] [CrossRef]
- Vialatte, F.B.; Maurice, M.; Dauwels, J.; Cichocki, A. Steady-State Visually Evoked Potentials: Focus on Essential Paradigms and Future Perspectives. Prog. Neurobiol. 2010, 90, 418–438. [Google Scholar] [CrossRef]
- Nijboer, F.; Furdea, A.; Gunst, I.; Mellinger, J.; McFarland, D.J.; Birbaumer, N.; Kübler, A. An Auditory Brain-Computer Interface (BCI). J. Neurosci. Methods 2008, 167, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Gao, X.; Hong, B.; Gao, S.; Wang, Y.; Gao, X.; Hong, B. Digital Object Identifier Visual and Auditory Brain-Computer Interfaces. IEEE Trans. Biomed. Eng. 2011, 61, 1436–1447. [Google Scholar] [CrossRef]
- Hill, N.J.; Lal, T.N.; Bierig, K.; Birbaumer, N.; Schölkopf, B. An Auditory Paradigm for Brain-Computer Interfaces. In Proceedings of the Advances in Neural Information Processing Systems, Vancouver, BC, Canada, 13–18 December 2004; Volume 17. [Google Scholar]
- Brouwer, A.M.; van Erp, J.B.F. A Tactile P300 Brain-Computer Interface. Front. Neurosci. 2010, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Punsawad, Y.; Wongsawat, Y. Motion Visual Stimulus for SSVEP-Based BCI System. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, San Diego, CA, USA, 28 August–1 September 2012; pp. 3837–3840. [Google Scholar]
- Thurlings, M.E.; Brouwer, A.M.; van Erp, J.B.F.; Werkhoven, P. Gaze-Independent ERP-BCIs: Augmenting Performance through Location-Congruent Bimodal Stimuli. Front. Syst. Neurosci. 2014, 8, 143. [Google Scholar] [CrossRef]
- Ortner, R.; Lugo, Z.; Noirhomme, Q.; Laureys, S.; Guger, C. A Tactile Brain-Computer Interface for Severely Disabled Patients. In Proceedings of the 2014 IEEE Haptics Symposium (HAPTICS), Houston, TX, USA, 23–26 February 2014; pp. 235–237. [Google Scholar]
- Ahn, S.; Kim, K.; Jun, S.C. Steady-State Somatosensory Evoked Potential for Brain-Computer Interface-Present and Future. Front. Hum. Neurosci. 2016, 9, 716. [Google Scholar] [CrossRef]
- Kaufmann, T.; Herweg, A.; Kübler, A. Toward Brain-Computer Interface Based Wheelchair Control Utilizing Tactually-Evoked Event-Related Potentials. J. Neuroeng. Rehabil. 2014, 11, 7. [Google Scholar] [CrossRef]
- Arpaia, P.; Coyle, D.; Donnarumma, F.; Esposito, A.; Natalizio, A.; Parvis, M. Visual and Haptic Feedback in Detecting Motor Imagery within a Wearable Brain–Computer Interface. Measurement 2023, 206, 112304. [Google Scholar] [CrossRef]
- Josiassen, R.C.; Shagass, C.; Roemer, R.A.; Ercegovac, D.V.; Straumanis, J.J. Somatosensory Evoked Potential Changes with a Selective Attention Task. Psychophysiology 1982, 19, 146–159. [Google Scholar] [CrossRef]
- Abiri, R.; Borhani, S.; Sellers, E.W.; Jiang, Y.; Zhao, X. A Comprehensive Review of EEG-Based Brain-Computer Interface Paradigms. J. Neural Eng. 2019, 16, 011001. [Google Scholar] [CrossRef]



| sERP Component | Search Window [ms] |
|---|---|
| P100 | 60–140 |
| N140 | 110–200 |
| P3a | 200–280 |
| P3b | 280–400 |
| Channel | ADSD | AVSD | Statistics D | AVSV | ADSV | Statistics V | ||
|---|---|---|---|---|---|---|---|---|
| P100 component | C3 | Peak [µV] | 1.47 ± 1.33 | 1.14 ± 1.21 | p = 0.244 | 1.68 ± 0.79 | 1.56 ± 0.88 | p = 0.685 |
| Latency [ms] | 101.21 ± 28.95 | 100.23 ± 29.74 | p = 0.520 | 102.35 ± 32.18 | 95.61 ± 29.63 | p = 0.148 | ||
| CP3 | Peak [µV] | 2.15 ± 1.08 | 1.88 ± 1.34 | p = 0.273 | 1.99 ± 1.12 | 2.15 ± 0.72 | p = 0.414 | |
| Latency [ms] | 91.21 ± 27.35 | 84.39 ± 23.56 | p = 0.157 | 90.61 ± 26.53 | 83.71 ± 24.30 | p = 0.201 | ||
| P3 | Peak [µV] | 2.29 ± 1.65 | 1.88 ± 1.44 | p = 0.340 | 2.79 ± 1.22 | 2.32 ± 1.06 | p = 0.273 | |
| Latency [ms] | 121.52 ± 21.35 | 117.73 ± 27.58 | p = 0.910 | 121.82 ± 22.70 | 121.52 ± 26.23 | p = 0.678 | ||
| Cz | Peak [µV] | 2.26 ± 1.33 | 1.91 ± 1.63 | p = 0.191 | 1.99 ± 1.33 | 2.14 ± 1.02 | p = 0.497 | |
| Latency [ms] | 84.92 ± 22.66 | 77.73 ± 16.85 | p = 0.096 | 86.74 ± 21.66 | 82.27 ± 17.15 | p = 0.850 | ||
| C4 | Peak [µV] | 1.22 ± 1.37 | 1.26 ± 1.19 | p = 0.839 | 1.54 ± 0.87 | 1.57 ± 0.73 | p = 1.000 | |
| Latency [ms] | 108.33 ± 20.90 | 105.83 ± 19.17 | p = 0.985 | 106.29 ± 25.65 | 99.24 ± 23.63 | p = 0.413 | ||
| N140 component | C3 | Peak [µV] | −0.14 ± 1.37 | −0.80 ± 1.31 | p = 0.048 | −0.04 ± 1.29 | −0.69 ± 1.59 | p = 0.048 |
| Latency [ms] | 140.08 ± 21.73 | 139.92 ± 27.66 | p = 0.904 | 138.94 ± 27.00 | 146.06 ± 28.44 | p = 0.168 | ||
| CP3 | Peak [µV] | −0.73 ± 1.23 | −1.38 ± 1.40 | p = 0.080 | −0.46 ± 1.17 | −0.95 ± 1.30 | p = 0.057 | |
| Latency [ms] | 139.70 ± 22.61 | 139.32 ± 25.39 | p = 0.814 | 142.80 ± 25.59 | 149.92 ± 24.07 | p = 0.339 | ||
| P3 | Peak [µV] | 0.62 ± 1.45 | −0.29 ± 1.41 | p = 0.006 | 1.23 ± 1.38 | −0.09 ± 1.55 | p = 0.002 | |
| Latency [ms] | 143.64 ± 25.20 | 154.47 ± 33.85 | p = 0.037 | 150.00 ± 30.97 | 161.06 ± 29.81 | p = 0.492 | ||
| Cz | Peak [µV] | −0.79 ± 1.12 | −1.35 ± 1.54 | p = 0.080 | −0.65 ± 1.24 | −1.11 ± 1.20 | p = 0.040 | |
| Latency [ms] | 149.09 ± 24.10 | 154.55 ± 30.57 | p = 0.227 | 157.42 ± 26.25 | 153.56 ± 22.50 | p = 0.765 | ||
| C4 | Peak [µV] | −0.34 ± 1.04 | −0.88 ± 1.04 | p = 0.094 | 0.19 ± 0.92 | −0.70 ± 1.19 | p = 0.008 | |
| Latency [ms] | 148.48 ± 24.53 | 160.38 ± 29.22 | p = 0.020 | 162.50 ± 24.74 | 167.50 ± 26.43 | p = 0.276 | ||
| P3a component | C3 | Peak [µV] | 3.44 ± 1.94 | 2.21 ± 1.82 | p = 0.002 | 3.57 ± 1.24 | 2.57 ± 1.09 | p = 0.002 |
| Latency [ms] | 243.86 ± 24.51 | 239.70 ± 24.92 | p = 0.128 | 257.65 ± 17.22 | 252.12 ± 28.89 | p = 0.918 | ||
| CP3 | Peak [µV] | 2.82 ± 1.75 | 1.59 ± 1.45 | p = 0.003 | 2.96 ± 0.90 | 2.16 ± 0.88 | p = 0.033 | |
| Latency [ms] | 249.47 ± 21.67 | 240.00 ± 26.78 | p = 0.182 | 261.82 ± 19.65 | 252.50 ± 29.58 | p = 0.359 | ||
| P3 | Peak [µV] | 4.14 ± 2.48 | 2.53 ± 2.24 | p = 0.001 | 4.30 ± 1.73 | 2.88 ± 1.61 | p = 0.000 | |
| Latency [ms] | 246.29 ± 23.81 | 245.91 ± 23.90 | p = 1.000 | 260.30 ± 19.35 | 252.27 ± 30.08 | p = 0.496 | ||
| Cz | Peak [µV] | 2.18 ± 1.61 | 0.78 ± 1.25 | p = 0.003 | 2.16 ± 0.70 | 1.55 ± 0.73 | p = 0.168 | |
| Latency [ms] | 251.52 ± 22.90 | 239.85 ± 29.51 | p = 0.278 | 261.59 ± 20.60 | 252.12 ± 30.79 | p = 0.383 | ||
| C4 | Peak [µV] | 2.48 ± 1.56 | 1.37 ± 1.35 | p = 0.017 | 2.79 ± 1.06 | 1.83 ± 1.05 | p = 0.003 | |
| Latency [ms] | 244.17 ± 22.47 | 232.58 ± 27.57 | p = 0.185 | 262.05 ± 20.10 | 250.98 ± 31.26 | p = 0.164 | ||
| P3b component | C3 | Peak [µV] | 3.18 ± 2.00 | 1.98 ± 1.53 | p= 0.002 | 3.52 ± 1.52 | 2.34 ± 1.63 | p = 0.001 |
| Latency [ms] | 318.79 ± 26.30 | 333.71 ± 36.45 | p = 0.014 | 319.24 ± 39.40 | 318.11 ± 33.16 | p = 0.922 | ||
| CP3 | Peak [µV] | 2.75 ± 1.61 | 1.42 ± 1.36 | p = 0.001 | 3.05 ± 1.26 | 1.73 ± 1.28 | p = 0.001 | |
| Latency [ms] | 315.76 ± 22.81 | 332.65 ± 35.17 | p = 0.007 | 318.79 ± 41.63 | 326.59 ± 33.71 | p = 0.677 | ||
| P3 | Peak [µV] | 3.34 ± 1.98 | 2.02 ± 1.53 | p = 0.001 | 3.98 ± 1.53 | 2.57 ± 1.73 | p = 0.002 | |
| Latency [ms] | 310.68 ± 25.22 | 324.62 ± 37.09 | p = 0.006 | 325.45 ± 46.39 | 327.20 ± 33.06 | p = 0.850 | ||
| Cz | Peak [µV] | 2.27 ± 1.27 | 0.88 ± 1.27 | p = 0.002 | 2.49 ± 1.01 | 1.18 ± 1.14 | p = 0.001 | |
| Latency [ms] | 328.86 ± 26.24 | 337.73 ± 39.11 | p = 0.349 | 341.97 ± 34.71 | 327.80 ± 34.20 | p = 0.077 | ||
| C4 | Peak [µV] | 2.10 ± 1.50 | 1.37 ± 1.30 | p = 0.027 | 3.09 ± 1.29 | 1.98 ± 1.73 | p = 0.008 | |
| Latency [ms] | 335.00 ± 31.20 | 339.47 ± 39.57 | p = 0.233 | 343.71 ± 37.24 | 345.23 ± 37.16 | p = 0.866 | ||
| Channel | ADSD | AVSD | Statistics D | AVSV | ADSV | Statistics V | ||
|---|---|---|---|---|---|---|---|---|
| P100 component | C3 | Area | 0.10 ± 0.05 | 0.08 ± 0.04 | p = 0.216 | 0.08 ± 0.03 | 0.09 ± 0.04 | p = 0.635 |
| CP3 | 0.11 ± 0.03 | 0.11 ± 0.04 | p = 0.893 | 0.10 ± 0.04 | 0.10 ± 0.04 | p = 0.787 | ||
| P3 | 0.13 ± 0.09 | 0.12 ± 0.06 | p = 0.839 | 0.13 ± 0.07 | 0.11 ± 0.06 | p = 0.216 | ||
| Cz | 0.11 ± 0.05 | 0.12 ± 0.05 | p = 0.414 | 0.11 ± 0.05 | 0.10 ± 0.05 | p = 0.414 | ||
| C4 | 0.09 ± 0.06 | 0.09 ± 0.05 | p = 1.000 | 0.10 ± 0.04 | 0.08 ± 0.04 | p = 0.168 | ||
| N140 component | C3 | Area | 0.11 ± 0.07 | 0.10 ± 0.05 | p = 0.146 | 0.11 ± 0.08 | 0.11 ± 0.07 | p = 0.040 |
| CP3 | 0.09 ± 0.04 | 0.09 ± 0.06 | p = 0.305 | 0.08 ± 0.06 | 0.10 ± 0.06 | p = 0.080 | ||
| P3 | 0.15 ± 0.10 | 0.12 ± 0.08 | p = 0.040 | 0.18 ± 0.11 | 0.14 ± 0.07 | p = 0.005 | ||
| Cz | 0.08 ± 0.05 | 0.09 ± 0.06 | p = 0.168 | 0.08 ± 0.04 | 0.08 ± 0.05 | p = 0.048 | ||
| C4 | 0.08 ± 0.05 | 0.07 ± 0.03 | p = 0.414 | 0.08 ± 0.05 | 0.08 ± 0.04 | p = 0.010 | ||
| P3a component | C3 | Area | 0.21 ± 0.14 | 0.16 ± 0.10 | p = 0.080 | 0.22 ± 0.08 | 0.14 ± 0.07 | p = 0.002 |
| CP3 | 0.17 ± 0.13 | 0.12 ± 0.08 | p = 0.080 | 0.17 ± 0.05 | 0.11 ± 0.05 | p = 0.008 | ||
| P3 | 0.25 ± 0.17 | 0.16 ± 0.13 | p = 0.000 | 0.26 ± 0.10 | 0.16 ± 0.09 | p = 0.000 | ||
| Cz | 0.13 ± 0.10 | 0.09 ± 0.06 | p = 0.094 | 0.11 ± 0.05 | 0.08 ± 0.04 | p = 0.080 | ||
| C4 | 0.14 ± 0.10 | 0.09 ± 0.07 | p = 0.080 | 0.15 ± 0.06 | 0.12 ± 0.04 | p = 0.021 | ||
| P3b component | C3 | Area | 0.26 ± 0.23 | 0.17 ± 0.13 | p = 0.006 | 0.32 ± 0.19 | 0.22 ± 0.17 | p = 0.001 |
| CP3 | 0.22 ± 0.18 | 0.15 ± 0.09 | p = 0.017 | 0.27 ± 0.15 | 0.18 ± 0.10 | p = 0.002 | ||
| P3 | 0.27 ± 0.23 | 0.16 ± 0.11 | p = 0.021 | 0.36 ± 0.19 | 0.24 ± 0.16 | p = 0.002 | ||
| Cz | 0.18 ± 0.13 | 0.12 ± 0.08 | p = 0.040 | 0.22 ± 0.11 | 0.14 ± 0.08 | p = 0.021 | ||
| C4 | 0.18 ± 0.17 | 0.12 ± 0.09 | p = 0.048 | 0.28 ± 0.16 | 0.18 ± 0.15 | p = 0.005 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novičić, M.; Savić, A.M. Somatosensory Event-Related Potential as an Electrophysiological Correlate of Endogenous Spatial Tactile Attention: Prospects for Electrotactile Brain-Computer Interface for Sensory Training. Brain Sci. 2023, 13, 766. https://doi.org/10.3390/brainsci13050766
Novičić M, Savić AM. Somatosensory Event-Related Potential as an Electrophysiological Correlate of Endogenous Spatial Tactile Attention: Prospects for Electrotactile Brain-Computer Interface for Sensory Training. Brain Sciences. 2023; 13(5):766. https://doi.org/10.3390/brainsci13050766
Chicago/Turabian StyleNovičić, Marija, and Andrej M. Savić. 2023. "Somatosensory Event-Related Potential as an Electrophysiological Correlate of Endogenous Spatial Tactile Attention: Prospects for Electrotactile Brain-Computer Interface for Sensory Training" Brain Sciences 13, no. 5: 766. https://doi.org/10.3390/brainsci13050766