An Assessment of the Pathological Classification and Postoperative Outcome of Focal Cortical Dysplasia by Simultaneous Hybrid PET/MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Plan and Demographic Characteristics
2.2. Inclusion and Exclusion Criteria of FCD Patients
2.3. Assessment of Clinical Data of FCD Patients
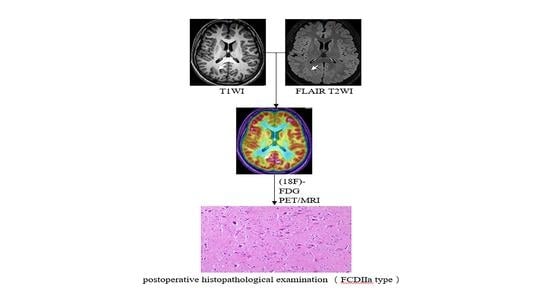
2.4. Histopathological Assessment of Patients with FCD
2.5. Obtainment and Assessment of the Simultaneous Hybrid PET/MRI and MRI Images
2.6. Statistical Analysis
3. Results
3.1. Assessment of Population Data of Patients with FCD
3.2. Comparison of MRI Images of FCD Patients
3.3. Comparison of FCD Patients with Preoperative Simultaneous Hybrid PET/MRI Findings
3.4. FCD Pathological Typing Analyses of MRI and Simultaneous Hybrid PET/MRI Findings
4. Discussion
4.1. Assessment of Clinical Data
4.2. Assessment of Preoperative MRI and Simultaneous Hybrid PET/MRI Findings
4.3. Assessment of MRI and Simultaneous Hybrid PET/MRI Findings by FCD Pathological Classification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Deng, D.; Zhou, C.; Li, H.; Guan, X.; Fang, L.; Cai, Q.; Wang, W.; Zhou, Q. Focal Cortical Dysplasia Type Related Medically Refractory Epilepsy: MRI Findings and Potential Predictors of Surgery Outcome. Diagnostics 2021, 11, 2225. [Google Scholar] [CrossRef]
- Kun, Y.; Zejun, D.; Jian, Z.; Feng, Z.; Changqing, L.; Xueling, Q. Surgical histopathologic findings of 232 Chinese children cases with drug-resistant seizures. Brain Behav. 2020, 10, e01565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbach, H.; Kellner, E.; Kremers, N.; Blumcke, I.; Demerath, T. MRI of focal cortical dysplasia. Neuroradiology 2022, 64, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Blumcke, I.; Thom, M.; Aronica, E.; Armstrong, D.D.; Vinters, H.V.; Palmini, A.; Jacques, T.S.; Avanzini, G.; Barkovich, A.J.; Battaglia, G.; et al. The clinicopathologic spectrum of focal cortical dysplasias: A consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 2011, 52, 158–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, M.J.; Choi, S.J.; Joo, E.Y.; Shon, Y.M.; Seo, D.W.; Hong, S.B.; Hong, S.C. Surgical outcome and prognostic factors in epilepsy patients with MR-negative focal cortical dysplasia. PLoS ONE 2021, 16, e0249929. [Google Scholar] [CrossRef]
- Rondinoni, C.; Magnun, C.; Vallota da Silva, A.; Heinsen, H.M.; Amaro, E., Jr. Epilepsy under the scope of ultra-high field MRI. Epilepsy Behav. 2021, 121 Pt B, 106366. [Google Scholar] [CrossRef]
- Halac, G.; Delil, S.; Zafer, D.; Isler, C.; Uzan, M.; Comunoglu, N.; Oz, B.; Yeni, S.N.; Vatankulu, B.; Halac, M.; et al. Compatibility of MRI and FDG-PET findings with histopathological results in patients with focal cortical dysplasia. Seizure 2017, 45, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Desarnaud, S.; Mellerio, C.; Semah, F.; Laurent, A.; Landre, E.; Devaux, B.; Chiron, C.; Lebon, V.; Chassoux, F. (18)F-FDG PET in drug-resistant epilepsy due to focal cortical dysplasia type 2: Additional value of electroclinical data and coregistration with MRI. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1449–1460. [Google Scholar] [CrossRef]
- Sun, K.; Ren, Z.; Yang, D.; Wang, X.; Yu, T.; Ni, D.; Qiao, L.; Xu, C.; Gao, R.; Lin, Y.; et al. Voxel-based morphometric MRI post-processing and PET/MRI co-registration reveal subtle abnormalities in cingulate epilepsy. Epilepsy Res. 2021, 171, 106568. [Google Scholar] [CrossRef]
- Hou, Y.; Guo, K.; Fan, X.; Shang, K.; Wang, J.; Wang, Z.; Shan, Y.; Zhao, G.; Lu, J. Crossed cerebellar diaschisis: Risk factors and prognostic value in focal cortical dysplasia by (18)F-FDG PET/CT. Ann. Nucl. Med. 2021, 35, 719–727. [Google Scholar] [CrossRef]
- Kikuchi, K.; Togao, O.; Yamashita, K.; Momosaka, D.; Nakayama, T.; Kitamura, Y.; Kikuchi, Y.; Baba, S.; Sagiyama, K.; Ishimatsu, K.; et al. Diagnostic accuracy for the epileptogenic zone detection in focal epilepsy could be higher in FDG-PET/MRI than in FDG-PET/CT. Eur. Radiol. 2021, 31, 2915–2922. [Google Scholar] [CrossRef]
- Martin, O.; Schaarschmidt, B.M.; Kirchner, J.; Suntharalingam, S.; Grueneisen, J.; Demircioglu, A.; Heusch, P.; Quick, H.H.; Forsting, M.; Antoch, G.; et al. PET/MRI Versus PET/CT for Whole-Body Staging: Results from a Single-Center Observational Study on 1,003 Sequential Examinations. J. Nucl. Med. 2020, 61, 1131–1136. [Google Scholar] [CrossRef]
- Pyatigorskaya, N.; Habert, M.O.; Rozenblum, L. Contribution of PET-MRI in brain diseases in clinical practice. Curr. Opin. Neurol. 2020, 33, 430–438. [Google Scholar] [CrossRef]
- De Muth, J.E. Overview of biostatistics used in clinical research. Am. J. Health Syst. Pharm. 2009, 66, 70–81. [Google Scholar] [CrossRef]
- Racz, A.; Becker, A.J.; Quesada, C.M.; Borger, V.; Vatter, H.; Surges, R.; Elger, C.E. Post-Surgical Outcome and Its Determining Factors in Patients Operated on With Focal Cortical Dysplasia Type II-A Retrospective Monocenter Study. Front. Neurol. 2021, 12, 666056. [Google Scholar] [CrossRef]
- Toth, M.; Barsi, P.; Toth, Z.; Borbely, K.; Luckl, J.; Emri, M.; Repa, I.; Janszky, J.; Doczi, T.; Horvath, Z.; et al. The role of hybrid FDG-PET/MRI on decision-making in presurgical evaluation of drug-resistant epilepsy. BMC Neurol. 2021, 21, 363. [Google Scholar] [CrossRef]
- Tahta, A.; Turgut, M. Focal cortical dysplasia: Etiology, epileptogenesis, classification, clinical presentation, imaging, and management. Childs Nerv. Syst. 2020, 36, 2939–2947. [Google Scholar] [CrossRef]
- Lee, W.S.; Baldassari, S.; Stephenson, S.E.M.; Lockhart, P.J.; Baulac, S.; Leventer, R.J. Cortical Dysplasia and the mTOR Pathway: How the Study of Human Brain Tissue Has Led to Insights into Epileptogenesis. Int. J. Mol. Sci. 2022, 23, 1344. [Google Scholar] [CrossRef]
- Coras, R.; Holthausen, H.; Sarnat, H.B. Focal cortical dysplasia type 1. Brain Pathol. 2021, 31, e12964. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Li, L.; Ren, L.; Wang, Y. Histological type of focal cortical dysplasia is associated with the risk of postsurgical seizure in children and adolescents. Ther. Clin. Risk Manag. 2019, 15, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Guerrini, R.; Barba, C. Focal cortical dysplasia: An update on diagnosis and treatment. Expert. Rev. Neurother. 2021, 21, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Thamcharoenvipas, T.; Takahashi, Y.; Kimura, N.; Matsuda, K.; Usui, N. Localizing and Lateralizing Value of Seizure Onset Pattern on Surface EEG in FCD Type II. Pediatr. Neurol. 2022, 129, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.S.; Liu, Q.Z.; Liu, M.; Zhang, Q.; Wang, R.F.; Wu, C.W.; Zhang, J.; Wang, W.; Ji, T.Y.; Liu, X.Y.; et al. Clinical features and surgical outcomes in young children with focal cortical dysplasia type II. CNS Neurosci. Ther. 2020, 26, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Zhang, X.; Zhang, G.; Yan, X.; Ma, K.; Qiao, L.; Wang, X.; Zhang, X.; Yu, T.; Wang, Y.; et al. Altered ripple density inside seizure onset zone in patients with focal cortical dysplasia-associated epilepsy. Brain Behav. 2021, 11, e02169. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.J.; Hu, W.H.; Wang, X.; Zhang, C.; Wang, S.S.; Zheng, Z.; Zhou, F.; Sang, L.; Zhang, K.; Zhang, J.G.; et al. Interictal pattern on scalp electroencephalogram predicts excellent surgical outcome of epilepsy caused by focal cortical dysplasia. Epilepsia Open 2022, 7, 350–360. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Vooturi, S.; Vadapalli, R.; Madigubba, S.; Panigrahi, M. Predictors of surgical outcome in focal cortical dysplasia and its subtypes. J. Neurosurg. 2021, 136, 512–522. [Google Scholar] [CrossRef]
- Truong, N.D.; Nguyen, A.D.; Kuhlmann, L.; Bonyadi, M.R.; Yang, J.; Ippolito, S.; Kavehei, O. Convolutional neural networks for seizure prediction using intracranial and scalp electroencephalogram. Neural Netw. 2018, 105, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lizana, E.; Fauser, S.; Brandt, A.; Schuler, E.; Wiegand, G.; Doostkam, S.; San Antonio-Arce, V.; Jacobs, J.; Bast, T.; Shah, M.; et al. Long-term seizure outcome in pediatric patients with focal cortical dysplasia undergoing tailored and standard surgical resections. Seizure 2018, 62, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Jayalakshmi, S.; Nanda, S.K.; Vooturi, S.; Vadapalli, R.; Sudhakar, P.; Madigubba, S.; Panigrahi, M. Focal Cortical Dysplasia and Refractory Epilepsy: Role of Multimodality Imaging and Outcome of Surgery. AJNR Am. J. Neuroradiol. 2019, 40, 892–898. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, D.; Qi, Y.; Qian, R.; Niu, C.; Fei, X. Surgical resection of dysembryoplatic neuroepithelioma tumor associated with epilepsy based on imaging classification. Neurol. Res. 2022, 44, 591–597. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, S.; Medrano, S.; Capellades, J.; Vilas, M.; Mestre, A.; Serrano, L.; Conesa, G.; Perez-Enriquez, C.; Arumi, M.; Bargallo, N.; et al. Voxel-based morphometry for the evaluation of patients with pharmacoresistant epilepsy with apparently normal MRI. J. Neuroimaging 2021, 31, 560–568. [Google Scholar] [CrossRef]
- Ganji, Z.; Hakak, M.A.; Zamanpour, S.A.; Zare, H. Automatic Detection of Focal Cortical Dysplasia Type II in MRI: Is the Application of Surface-Based Morphometry and Machine Learning Promising? Front. Hum. Neurosci. 2021, 15, 608285. [Google Scholar] [CrossRef]
- Isler, C.; Ozkara, C.; Kucukyuruk, B.; Delil, S.; Oz, B.; Comunoglu, N.; Kizilkilic, O.; Kayhan, A.; Deniz, K.; Akkol, S.; et al. Seizure Outcome of Patients with Magnetic Resonance Imaging-Negative Epilepsies: Still An Ongoing Debate. World Neurosurg. 2017, 106, 638–644. [Google Scholar] [CrossRef]
- Guo, K.; Cui, B.; Shang, K.; Hou, Y.; Fan, X.; Yang, H.; Zhao, G.; Lu, J. Assessment of localization accuracy and postsurgical prediction of simultaneous (18)F-FDG PET/MRI in refractory epilepsy patients. Eur. Radiol. 2021, 31, 6974–6982. [Google Scholar] [CrossRef]
- Guo, K.; Wang, J.; Cui, B.; Wang, Y.; Hou, Y.; Zhao, G.; Lu, J. [18F]FDG PET/MRI and magnetoencephalography may improve presurgical localization of temporal lobe epilepsy. Eur. Radiol. 2022, 32, 3024–3034. [Google Scholar] [CrossRef]
- Niu, N.; Xing, H.; Wu, M.; Ma, Y.; Liu, Y.; Ba, J.; Zhu, S.; Li, F.; Huo, L. Performance of PET imaging for the localization of epileptogenic zone in patients with epilepsy: A meta-analysis. Eur. Radiol. 2021, 31, 6353–6366. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, Y.; Jiang, B.; Zhou, Y.; Jin, B.; Hou, H.; Wu, S.; Zhu, J.; Wang, Z.I.; Wong, C.H.; et al. 18F-FDG PET and high-resolution MRI co-registration for pre-surgical evaluation of patients with conventional MRI-negative refractory extra-temporal lobe epilepsy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1567–1572. [Google Scholar] [CrossRef]


| Projects | Gender | Classification of FCD | ||||||
|---|---|---|---|---|---|---|---|---|
| All FCD Patients (n = 69) | Female (n = 30) | Male (n = 39) | p Value | Type Ⅰ FCD (n = 3) | Type Ⅱ FCD (n = 25) | Type Ⅲ FCD (n = 41) | p Value | |
| ASO | 16.64 ± 12.86 | 15.93 ± 13.65 | 17.18 ± 12.36 | 0.693 | 9.67 ± 6.51 | 12.04 ± 8.87 | 19.95 ± 12.27 | 0.058 |
| Duration of epilepsy | 8.83 ± 7.03 | 10.28 ± 7.45 | 7.71 ± 6.58 | 0.128 | 13.33 ± 5.77 | 9.80 ± 7.72 | 7.90 ± 6.61 | 0.221 |
| Age at surgery | 25.46 ± 12.96 | 26.21 ± 13.88 | 24.89 ± 12.35 | 0.677 | 23.00 ± 8.89 | 21.84 ± 10.10 | 27.85 ± 14.35 | 0.252 |
| Age of Seizure Onset | Age at Surgery | |||||
|---|---|---|---|---|---|---|
| <18 years (n = 44) | >18 years (n = 25) | p value | <18 years (n = 22) | >18 years (n = 47) | p value | |
| ASO | 8.76 (0–18) | 30.50 (19–55) | - | 5.72 (0–14) | 21.75 (1–55) | - |
| Duration of epilepsy | 9.60 (0.7–28) | 7.45 (0.02–23) | 0.036 | 5.83 (0.7–14) | 10.23 (0.02–28) | 0.021 |
| Age at surgery | 18.36 (5–36) | 37.96 (22–62) | - | 11.55 (6–17) | 31.98 (19–62) | - |
| FCD type | 0.106 | 0.817 | ||||
| Type I FCD | 3 (7%) | 0 | 1 (4%) | 2 (4%) | ||
| Type II FCD | 19 (43%) | 6 (24%) | 9 (41%) | 16 (34%) | ||
| Type III FCD | 22 (50%) | 19 (76%) | 12 (55%) | 29 (62%) | ||
| MRI findings | 0.064 | 0.340 | ||||
| MRI-positive | 24 (54.55%) | 19 (76.00%) | 15 (68.18%) | 28 (59.57%) | ||
| MRI-negative | 20 (45.45%) | 6 (24.00%) | 7 (31.82%) | 19 (40.43%) | ||
| Simultaneous Hybrid PET/MRI findings | 0.734 | 0.103 | ||||
| Simultaneous Hybrid PET/MRI-positive | 36 (81.82%) | 22 (88.00%) | 21 (95.46%) | 38 (80.86%) | ||
| Simultaneous Hybrid PET/MRI-negative | 8 (18.18%) | 3 (12.00%) | 1 (4.54%) | 9 (19.14%) | ||
| MRI-Positive (n = 43) | MRI-Negative (n = 26) | p Value | Simultaneous Hybrid PET/MRI-Positive (n = 59) | Simultaneous Hybrid PET/MRI-Negative (n = 10) | p Value | |
|---|---|---|---|---|---|---|
| FCD type | 0.003 | 0.037 | ||||
| Type I FCD | 1 (2%) | 2 (8%) | 1 (2%) | 2 (20%) | ||
| Type II FCD | 10 (23%) | 15 (58%) | 23 (39%) | 2 (20%) | ||
| Type III FCD | 32 (74%) | 9 (34%) | 35 (59%) | 6 (60%) | ||
| FCD location | 0.135 | 0.073 | ||||
| Temporal | 13 (30%) | 12 (47%) | 21 (36%) | 4 (40%) | ||
| Frontal | 10 (23%) | 3 (13%) | 10 (17%) | 3 (30%) | ||
| Parietooccipital | 3 (7%) | 5 (19%) | 6 (10%) | 2 (20%) | ||
| Multilobar | 17 (40%) | 6 (21%) | 22 (37%) | 1 (10%) | ||
| Age at onset | 17.97 ± 13.20 | 14.44 ± 12.19 | 0.258 | 16.34 ± 12.53 | 18.40 ± 15.25 | 0.696 |
| Duration of epilepsy | 7.73 ± 6.16 | 10.64 ± 8.08 | 0.186 | 8.41 ± 6.78 | 11.30 ± 8.31 | 0.320 |
| Age at surgery | 25.70 ± 13.44 | 25.08 ± 12.36 | 0.489 | 24.74 ± 12.79 | 29.70 ± 13.81 | 0.378 |
| MRI-Positive/Simultaneous Hybrid PET/MRI-Positive (n = 43) | MRI-Positive/Simultaneous Hybrid PET/MRI-Negative (n = 0) | MRI-Negative/Simultaneous Hybrid PET/MRI-Positive (n = 16) | MRI-Negative/Simultaneous Hybrid PET/MRI-Negative (n = 10) | p Value | |
|---|---|---|---|---|---|
| Type I | 1 (2%) | - | - | 2 (20%) | 1.000 |
| Type II | 10 (23%) | - | 13 (81%) | 2 (20%) | 0.000 |
| Type III | 32 (74%) | - | 3 (19%) | 6 (60%) | 0.391 |
| Temporal | 13 (30%) | - | 8 (50%) | 4 (40%) | 0.005 |
| Frontal | 10 (23%) | - | - | 3 (30%) | 1.000 |
| Parietooccipital | 3 (7%) | - | 3 (19%) | 2 (20%) | 0.131 |
| Multilobar | 17 (40%) | - | 5 (31%) | 1 (10%) | 0.040 |
| Age at onset | 17.97 ± 13.20 | - | 11.96 ± 9.54 | 18.40 ± 15.25 | 0.254 |
| Duration of epilepsy | 7.73 ± 6.16 | - | 10.23 ± 8.17 | 11.30 ± 8.31 | 0.236 |
| Age at surgery | 25.70 ± 13.41 | - | 22.19 ± 10.82 | 29.70 ± 13.81 | 0.347 |
| Good Outcome | Poor Outcome | ||
|---|---|---|---|
| Engel I | Engel II | Engel III + IV | |
| MRI+/Simultaneous Hybrid PET/MRI+ (n = 43) | 30 | 9 | 4 |
| MRI+/Simultaneous Hybrid PET/MRI− (n = 0) | - | - | - |
| MRI−/Simultaneous Hybrid PET/MRI+ (n = 16) | 13 | 3 | - |
| MRI−/Simultaneous Hybrid PET/MRI− (n = 10) | 3 | 3 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Wang, L.; Yu, Y.; Li, G.; Cao, C.; Xu, R.; Jiang, B.; Bi, Y.; Xie, M.; Hu, C.; et al. An Assessment of the Pathological Classification and Postoperative Outcome of Focal Cortical Dysplasia by Simultaneous Hybrid PET/MRI. Brain Sci. 2023, 13, 611. https://doi.org/10.3390/brainsci13040611
Wang N, Wang L, Yu Y, Li G, Cao C, Xu R, Jiang B, Bi Y, Xie M, Hu C, et al. An Assessment of the Pathological Classification and Postoperative Outcome of Focal Cortical Dysplasia by Simultaneous Hybrid PET/MRI. Brain Sciences. 2023; 13(4):611. https://doi.org/10.3390/brainsci13040611
Chicago/Turabian StyleWang, Ning, Lingjie Wang, Yixing Yu, Guangzheng Li, Changhao Cao, Rui Xu, Bin Jiang, Yongfeng Bi, Minjia Xie, Chunhong Hu, and et al. 2023. "An Assessment of the Pathological Classification and Postoperative Outcome of Focal Cortical Dysplasia by Simultaneous Hybrid PET/MRI" Brain Sciences 13, no. 4: 611. https://doi.org/10.3390/brainsci13040611