Changes in the Intranetwork and Internetwork Connectivity of the Default Mode Network and Olfactory Network in Patients with COVID-19 and Olfactory Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Acquisition of MRI Data
2.3. Quantitative Assessment of Olfactory Function
2.4. Analysis of rs-fMRI Data
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report; World Health Organization: Geneva, Switzerland, 2022.
- Chiesa-Estomba, C.M.; Lechien, J.R.; Radulesco, T.; Michel, J.; Sowerby, L.J.; Hopkins, C.; Saussez, S. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur. J. Neurol 2020, 27, 2318–2321. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Surda, P.; Kumar, N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology 2020, 58, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Kandemirli, S.G.; Dogan, L.; Sarikaya, Z.T.; Kara, S.; Akinci, C.; Kaya, D.; Kaya, Y.; Yildirim, D.; Tuzuner, F.; Yildirim, M.S.; et al. Brain MRI Findings in Patients in the Intensive Care Unit with COVID-19 Infection. Radiology 2020, 297, E232–E235. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 2020, 77, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Soraas, A.; Bo, R.; Kalleberg, K.T.; Stoer, N.C.; Ellingjord-Dale, M.; Landro, N.I. Self-reported Memory Problems 8 Months After COVID-19 Infection. JAMA Netw. Open 2021, 4, e2118717. [Google Scholar] [CrossRef]
- Chung, T.W.; Zhang, H.; Wong, F.K.; Sridhar, S.; Chan, K.H.; Cheng, V.C.; Yuen, K.Y.; Hung, I.F.; Mak, H.K. Neurosensory Rehabilitation and Olfactory Network Recovery in COVID-19-related Olfactory Dysfunction. Brain Sci. 2021, 11, 686. [Google Scholar] [CrossRef]
- Kollndorfer, K.; Fischmeister, F.P.; Kowalczyk, K.; Hoche, E.; Mueller, C.A.; Trattnig, S.; Schopf, V. Olfactory training induces changes in regional functional connectivity in patients with long-term smell loss. NeuroImage Clin. 2015, 9, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Cecchetto, C.; Fischmeister, F.P.S.; Reichert, J.L.; Bagga, D.; Schopf, V. When to collect resting-state data: The influence of odor on post-task resting-state connectivity. NeuroImage 2019, 191, 361–366. [Google Scholar] [CrossRef]
- Mevel, K.; Chetelat, G.; Eustache, F.; Desgranges, B. The default mode network in healthy aging and Alzheimer’s disease. Int. J. Alzheimers Dis. 2011, 2011, 535816. [Google Scholar] [CrossRef] [Green Version]
- Schneider, F.; Bermpohl, F.; Heinzel, A.; Rotte, M.; Walter, M.; Tempelmann, C.; Wiebking, C.; Dobrowolny, H.; Heinze, H.J.; Northoff, G. The resting brain and our self: Self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience 2008, 157, 120–131. [Google Scholar] [CrossRef]
- Yeshurun, Y.; Nguyen, M.; Hasson, U. The default mode network: Where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 2021, 22, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Mitchell, D.J.; Duncan, J. The Functional Convergence and Heterogeneity of Social, Episodic, and Self-Referential Thought in the Default Mode Network. Cereb. Cortex 2020, 30, 5915–5929. [Google Scholar] [CrossRef] [PubMed]
- Gardini, S.; Venneri, A.; Sambataro, F.; Cuetos, F.; Fasano, F.; Marchi, M.; Crisi, G.; Caffarra, P. Increased functional connectivity in the default mode network in mild cognitive impairment: A maladaptive compensatory mechanism associated with poor semantic memory performance. J. Alzheimers Dis. 2015, 45, 457–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, M.; Jann, K.; Dierks, T.; Federspiel, A.; Wiest, R.; Horn, H. Semantic memory involvement in the default mode network: A functional neuroimaging study using independent component analysis. NeuroImage 2011, 54, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Scheibner, H.J.; Bogler, C.; Gleich, T.; Haynes, J.D.; Bermpohl, F. Internal and external attention and the default mode network. NeuroImage 2017, 148, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Karunanayaka, P.R.; Wilson, D.A.; Tobia, M.J.; Martinez, B.E.; Meadowcroft, M.D.; Eslinger, P.J.; Yang, Q.X. Default mode network deactivation during odor-visual association. Hum. Brain Mapp. 2017, 38, 1125–1139. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Yang, Q.X.; Zhang, H.; Eslinger, P.J.; Zhang, X.; Wu, S.; Zhang, B.; Zhu, B.; Karunanayaka, P.R. Disruptions of the olfactory and default mode networks in Alzheimer’s disease. Brain Behav. 2019, 9, e01296. [Google Scholar] [CrossRef]
- Mazziotta, J.C.; Woods, R.; Iacoboni, M.; Sicotte, N.; Yaden, K.; Tran, M.; Bean, C.; Kaplan, J.; Toga, A.W.; Members of the International Consortium for Brain Mapping. The myth of the normal, average human brain--the ICBM experience: (1) subject screening and eligibility. NeuroImage 2009, 44, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Yuen, K.-Y.; Hung, I.F.-N.; Lung, K.-C.; Cheng, V.C.-C.; To, K.K.-W.; Ng, P.Y.; Chan, W.-M.; Leung, S.-M.; Wu, A.K.-L.; Lam, S.H.-Y.; et al. Olfactory Dysfunction in Coronavirus Disease 2019 Patients: Observational Cohort Study and Systematic Review. Open Forum Infect. Dis. 2020, 7, ofaa199. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. NeuroImage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Friston, K.J.; Williams, S.; Howard, R.; Frackowiak, R.S.J.; Turner, R. Movement-Related effects in fMRI time-series. Magn. Reson. Med. 1996, 35, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Satterthwaite, T.D.; Elliott, M.A.; Gerraty, R.T.; Ruparel, K.; Loughead, J.; Calkins, M.E.; Eickhoff, S.B.; Hakonarson, H.; Gur, R.C.; Gur, R.E.; et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage 2013, 64, 240–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 2014, 84, 320–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, D.; Qin, W.; Li, Q.; Chen, B.; Zhang, Y.; Yu, C. Altered resting-state brain activity in obstructive sleep apnea. Sleep 2013, 36, 651–659. [Google Scholar] [CrossRef]
- Saive, A.L.; Royet, J.P.; Plailly, J. A review on the neural bases of episodic odor memory: From laboratory-based to autobiographical approaches. Front Behav. Neurosci. 2014, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Arnold, T.C.; You, Y.; Ding, M.; Zuo, X.N.; de Araujo, I.; Li, W. Functional Connectome Analyses Reveal the Human Olfactory Network Organization. eNeuro 2020, 7. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-anatomic fractionation of the brain’s default network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Hu, N.; Zhang, W.; Tao, B.; Dai, J.; Gong, Y.; Tan, Y.; Cai, D.; Lui, S. Dysconnectivity of Multiple Brain Networks in Schizophrenia: A Meta-Analysis of Resting-State Functional Connectivity. Front. Psychiatry 2019, 10, 482. [Google Scholar] [CrossRef] [Green Version]
- Islek, A.; Balci, M.K. Diagnostic Value of Butanol Threshold Test in COVID-19 Related Olfactory Dysfunction. Indian J. Otolaryngol. Head Neck Surg. 2021, 47, 1–5. [Google Scholar] [CrossRef]
- Kirschenbaum, D.; Imbach, L.L.; Ulrich, S.; Rushing, E.J.; Keller, E.; Reimann, R.R.; Frauenknecht, K.B.M.; Lichtblau, M.; Witt, M.; Hummel, T.; et al. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet 2020, 396, 166. [Google Scholar] [CrossRef]
- Conde Cardona, G.; Quintana Pajaro, L.D.; Quintero Marzola, I.D.; Ramos Villegas, Y.; Moscote Salazar, L.R. Neurotropism of SARS-CoV 2: Mechanisms and manifestations. J. Neurol. Sci. 2020, 412, 116824. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brunink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Poyiadji, N.; Shahin, G.; Noujaim, D.; Stone, M.; Patel, S.; Griffith, B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: Imaging Features. Radiology 2020, 296, E119–E120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zhang, M.; Wang, J.; Gao, J. Sars-Cov-2: Underestimated damage to nervous system. Travel Med. Infect. Dis. 2020, 36, 101642. [Google Scholar] [CrossRef]
- Carlson, H.; Leitao, J.; Delplanque, S.; Cayeux, I.; Sander, D.; Vuilleumier, P. Sustained effects of pleasant and unpleasant smells on resting state brain activity. Cortex 2020, 132, 386–403. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, X.; Yi, H.; Luo, Y.; Yan, Q.; Feng, T.; He, Q.; Lei, X.; Qiu, J.; Chen, H. Stronger functional network connectivity and social support buffer against negative affect during the COVID-19 outbreak and after the pandemic peak. Neurobiol. Stress 2021, 15, 100418. [Google Scholar] [CrossRef]
- Barulli, D.; Stern, Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Sheline, Y.I.; Morris, J.C.; Snyder, A.Z.; Price, J.L.; Yan, Z.; D’Angelo, G.; Liu, C.; Dixit, S.; Benzinger, T.; Fagan, A.; et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J. Neurosci. 2010, 30, 17035–17040. [Google Scholar] [CrossRef] [Green Version]
- Lind, J.; Persson, J.; Ingvar, M.; Larsson, A.; Cruts, M.; Van Broeckhoven, C.; Adolfsson, R.; Backman, L.; Nilsson, L.G.; Petersson, K.M.; et al. Reduced functional brain activity response in cognitively intact apolipoprotein E epsilon4 carriers. Brain 2006, 129, 1240–1248. [Google Scholar] [CrossRef]
- Wu, X.; Li, R.; Fleisher, A.S.; Reiman, E.M.; Guan, X.; Zhang, Y.; Chen, K.; Yao, L. Altered default mode network connectivity in Alzheimer’s disease—A resting functional MRI and Bayesian network study. Hum. Brain Mapp. 2011, 32, 1868–1881. [Google Scholar] [CrossRef] [Green Version]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Cirillo, M.; De Micco, R.; Caiazzo, G.; Siciliano, M.; Russo, A.G.; Monari, C.; Coppola, N.; Tedeschi, G.; Tessitore, A. Olfactory loss and brain connectivity after COVID-19. Hum. Brain Mapp. 2022, 43, 1548–1560. [Google Scholar] [CrossRef]
- Postma, E.M.; Smeets, P.A.M.; Boek, W.M.; Boesveldt, S. Investigating morphological changes in the brain in relation to etiology and duration of olfactory dysfunction with voxel-based morphometry. Sci. Rep. 2021, 11, 12704. [Google Scholar] [CrossRef] [PubMed]
- Kluczyński, Ł.; Janiak-Kiszka, J.; Kaźmierczak, W. The sense of smell in chronic rhinosinusitis—Literature review. Med. Res. J. 2018, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]





| Characteristics | Healthy Controls | COVID-19 Patients | p Value |
|---|---|---|---|
| N | 13 | 22 | - |
| Butanol threshold test (BTT) | - | 2.25 ± 1.09 | - |
| The University of Pennsylvania Smell identification test (UPSIT) | - | 23.6 ± 7.4 | - |
| OD-onset to MR scan (Days) | - | 164.2 ± 50.6 | - |
| SARS-CoV-2 diagnosis | negative | positive | - |
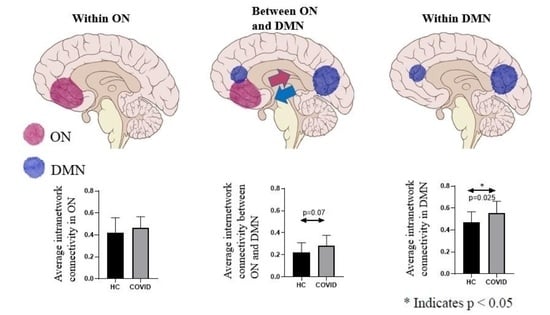
| Average intranetwork connectivity in ON | 0.42 ± 0.14 | 0.48 ± 0.11 | 0.20 |
| Average intranetwork connectivity in DMN | 0.49 ± 0.10 | 0.58 ± 0.10 | 0.013 * |
| Average internetwork connectivity between ON and DMN | 0.21 ± 0.09 | 0.28 ± 0.09 | 0.048 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chung, T.W.-H.; Wong, F.K.-C.; Hung, I.F.-N.; Mak, H.K.-F. Changes in the Intranetwork and Internetwork Connectivity of the Default Mode Network and Olfactory Network in Patients with COVID-19 and Olfactory Dysfunction. Brain Sci. 2022, 12, 511. https://doi.org/10.3390/brainsci12040511
Zhang H, Chung TW-H, Wong FK-C, Hung IF-N, Mak HK-F. Changes in the Intranetwork and Internetwork Connectivity of the Default Mode Network and Olfactory Network in Patients with COVID-19 and Olfactory Dysfunction. Brain Sciences. 2022; 12(4):511. https://doi.org/10.3390/brainsci12040511
Chicago/Turabian StyleZhang, Hui, Tom Wai-Hin Chung, Fergus Kai-Chuen Wong, Ivan Fan-Ngai Hung, and Henry Ka-Fung Mak. 2022. "Changes in the Intranetwork and Internetwork Connectivity of the Default Mode Network and Olfactory Network in Patients with COVID-19 and Olfactory Dysfunction" Brain Sciences 12, no. 4: 511. https://doi.org/10.3390/brainsci12040511