The Quantum Tunneling of Ions Model Can Explain the Pathophysiology of Tinnitus
Abstract
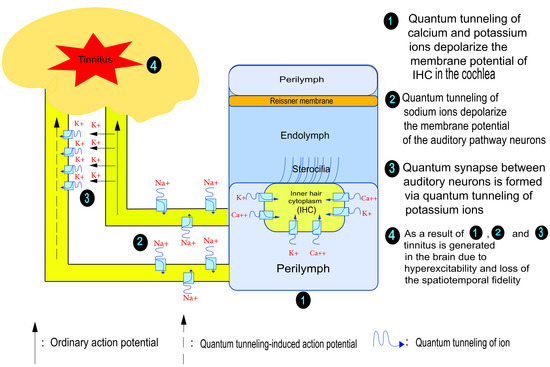
:1. Introduction
2. Mathematical Model of the Quantum Tunneling of Ions
2.1. Mathematical Equations That Describe the Quantum Tunneling-Induced Membrane Depolarization of Inner Hair Cells
2.2. Mathematical Equations That Describe the Probability of Inducing an Action Potential in Demyelinated Neurons of the Auditory Pathway (the Formation of a Quantum Synapse)
3. Results
3.1. Quantum Tunneling-Induced Membrane Depolarization
3.1.1. The Influence of the Length of the Gate on Quantum Tunneling-Induced Membrane Depolarization
3.1.2. The Influence of Gate Location on Quantum Tunneling-Induced Membrane Depolarization
3.2. The Probability of Action Potential Induction via Quantum Tunneling of Potassium Ions (the Formation of a Quantum Synapse)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haider, H.F.; Bojić, T.; Ribeiro, S.F.; Paço, J.; Hall, D.A.; Szczepek, A.J. Pathophysiology of subjective tinnitus: Triggers and mainte-nance. Front. Neurosci. 2018, 12, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.; El Refaie, A. Epidemiology of tinnitus. In Tinnitus Handbook (Singular Audiology Text); Singular Pub. Group: San Diego, CA, USA, 2000. [Google Scholar]
- Rojas, R.; Palacios, E.; D’Antonio, M.; Correa, G. Aberrant Internal Carotid Artery as a Cause of Pulsatile Tinnitus and an Intratympanic Mass. Ear Nose Throat J. 2003, 82, 173–174. [Google Scholar] [CrossRef]
- Noreña, A.J.; Farley, B. Tinnitus-related neural activity: Theories of generation, propagation, and centralization. Hear. Res. 2013, 295, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Noreña, A.J. Revisiting the Cochlear and Central Mechanisms of Tinnitus and Therapeutic Approaches. Audiol. Neurotol. 2015, 20 (Suppl. S1), 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hudspeth, A.J. The Cellular Basis of Hearing: The Biophysics of Hair Cells. Science 1985, 230, 745–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, T.; Neef, A.; Khimich, D. Mechanisms underlying the temporal precision of sound coding at the inner hair cell ribbon synapse. J. Physiol. 2006, 576, 55–62. [Google Scholar] [CrossRef]
- Parra, L.C.; Pearlmutter, B. Illusory percepts from auditory adaptation. J. Acoust. Soc. Am. 2007, 121, 1632–1641. [Google Scholar] [CrossRef] [Green Version]
- Patuzzi, R. Non-Linear Aspects of Outer Hair Cell Transduction and the Temporary Threshold Shifts after Acoustic Trauma. Audiol. Neurotol. 2002, 7, 17–20. [Google Scholar] [CrossRef]
- Douguet, D.; Honoré, E. Mammalian Mechanoelectrical Transduction: Structure and Function of Force-Gated ion Channels. Cell 2019, 179, 340–354. [Google Scholar] [CrossRef]
- Liberman, M.; Dodds, L.W. Single-neuron labeling and chronic cochlear pathology. II. Stereocilia damage and alterations of spontaneous discharge rates. Hear. Res. 1984, 16, 43–53. [Google Scholar] [CrossRef]
- Baguley, D.M. Mechanisms of tinnitus. Br. Med. Bull. 2002, 63, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Qaswal, A.B. Quantum tunneling of ions through the closed voltage-gated channels of the biological membrane: A mathe-matical model and implications. Quantum Rep. 2019, 1, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Qaswal, A.B.; Ababneh, O.; Khreesha, L.; Al-Ani, A.; Suleihat, A.; Abbad, M. Mathematical Modeling of ion Quantum Tunneling Reveals Novel Properties of Voltage-Gated Channels and Quantum Aspects of Their Pathophysiology in Excitability-Related Disorders. Pathophysiology 2021, 28, 116–154. [Google Scholar] [CrossRef]
- Qaswal, A.B. Quantum Electrochemical Equilibrium: Quantum Version of the Goldman–Hodgkin–Katz Equation. Quantum Rep. 2020, 2, 266–277. [Google Scholar] [CrossRef]
- Qaswal, A. A Theoretical Study to Explain the Referred Pain Phenomenon and Its Characteristics via Quantum Tunneling of Potassium Ions Through the Channels of Neuronal Membrane. NeuroQuantology 2019, 17, 43. [Google Scholar] [CrossRef]
- Qaswal, A.B. The myelin sheath maintains the spatiotemporal fidelity of action potentials by eliminating the effect of quantum tunneling of potassium ions through the closed channels of the neuronal membrane. Quantum Rep. 2019, 1, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Khreesha, L.; Qaswal, A.B.; Al Omari, B.; Albliwi, M.A.; Ababneh, O.; Albanna, A.; Abunab’ah, A.; Iswaid, M.; Alarood, S.; Guzu, H.; et al. Quantum Tunneling-Induced Membrane Depolarization Can Explain the Cellular Effects Mediated by Lithium: Mathematical Modeling and Hypothesis. Membranes 2021, 11, 851. [Google Scholar] [CrossRef]
- Alrabayah, M.; Qaswal, A.B.; Suleiman, A.; Khreesha, L. Role of Potassium Ions Quantum Tunneling in the Pathophysiology of Phantom Limb Pain. Brain Sci. 2020, 10, 241. [Google Scholar] [CrossRef] [Green Version]
- De Ridder, D.; Elgoyhen, A.B.; Romo, R.; Langguth, B. Phantom percepts: Tinnitus and pain as persisting aversive memory net-works. Proc. Natl. Acad. Sci. USA 2011, 108, 8075–8080. [Google Scholar] [CrossRef] [Green Version]
- Long, P.; Wan, G.; Roberts, M.; Corfas, G. Myelin development, plasticity, and pathology in the auditory system. Dev. Neurobiol. 2018, 78, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Aryal, P.; Sansom, M.S.; Tucker, S.J. Hydrophobic Gating in ion Channels. J. Mol. Biol. 2015, 427, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hering, S.; Zangerl-Plessl, E.-M.; Beyl, S.; Hohaus, A.; Andranovits, S.; Timin, E.N. Calcium channel gating. Pflügers Arch.-Eur. J. Physiol. 2018, 470, 1291–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oelstrom, K.; Goldschen-Ohm, M.P.; Holmgren, M.; Chanda, B. Evolutionarily conserved intracellular gate of voltage-dependent sodium channels. Nat. Commun. 2014, 5, 3420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labro, A.J.; Snyders, D.J. Being Flexible: The Voltage-Controllable Activation Gate of Kv Channels. Front. Pharmacol. 2012, 3, 168. [Google Scholar] [CrossRef] [Green Version]
- Payandeh, J.; Gamal El-Din, T.M.; Scheuer, T.; Zheng, N.; Catterall, W.A. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature 2012, 486, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Cuello, L.G.; Jogini, V.; Cortes, D.M.; Perozo, E. Structural mechanism of C-type inactivation in K+ channels. Nature 2010, 466, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Bagnéris, C.; Naylor, C.E.; Mc Cusker, E.C.; Wallace, B.A. Structural model of the open–closed–inactivated cycle of prokaryotic voltage-gated sodium channels. J. Gen. Physiol. 2014, 145, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.C.; Yang, H.; Liu, Z.; Sun, F. Thermodynamics of voltage-gated ion channels. Biophys. Rep. 2018, 4, 300–319. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Yazdani, M.; Zhang, G.; Cui, J.; Chen, J. Hydrophobic gating in BK channels. Nat. Commun. 2018, 9, 3408. [Google Scholar] [CrossRef]
- Aryal, P.; Abd-Wahab, F.; Bucci, G.; Sansom, M.S.P.; Tucker, S.J. A hydrophobic barrier deep within the inner pore of the TWIK-1 K2P potassium channel. Nat. Commun. 2014, 5, 4377. [Google Scholar] [CrossRef] [Green Version]
- Tepper, H.L.; Voth, G.A. Mechanisms of Passive ion Permeation through Lipid Bilayers: Insights from Simulations. J. Phys. Chem. B 2006, 110, 21327–21337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khavrutskii, I.V.; Gorfe, A.A.; Lu, B.; Mc Cammon, J.A. Free energy for the permeation of Na+ and Cl− ions and their ion-pair through a zwitterionic dimyristoyl phosphatidylcholine lipid bilayer by umbrella integration with harmonic fourier beads. J. Am. Chem. Soc. 2009, 131, 1706–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, A.K. Introductory Quantum Chemistry; Tata McGraw-Hill Education: New York, NY, USA, 1994. [Google Scholar]
- Eckart, C. The Penetration of a Potential Barrier by Electrons. Phys. Rev. (Ser. I) 1930, 35, 1303–1309. [Google Scholar] [CrossRef]
- Miyazaki, T. Atom Tunneling Phenomena in Physics, Chemistry and Biology; Springer Science & Business Media: Berlin, Germany, 2004. [Google Scholar]
- Rao, S.; Lynch, C.I.; Klesse, G.; Oakley, G.E.; Stansfeld, P.J.; Tucker, S.J.; Sansom, M.S.P. Water and hydrophobic gates in ion channels and nanopores. Faraday Discuss. 2018, 209, 231–247. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Klesse, G.; Stansfeld, P.J.; Tucker, S.J.; Sansom, M.S.P. A heuristic derived from analysis of the ion channel structural proteome permits the rapid identification of hydrophobic gates. Proc. Natl. Acad. Sci. USA 2019, 116, 13989–13995. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Klesse, G.; Lynch, C.I.; Tucker, S.J.; Sansom, M.S.P. Molecular Simulations of Hydrophobic Gating of Pentameric Ligand Gated ion Channels: Insights into Water and Ions. J. Phys. Chem. B 2021, 125, 981–994. [Google Scholar] [CrossRef]
- Köpfer, D.A.; Song, C.; Gruene, T.; Sheldrick, G.M.; Zachariae, U.; de Groot, B.L. Ion permeation in K + channels occurs by direct Coulomb knock-on. Science 2014, 346, 352–355. [Google Scholar] [CrossRef] [Green Version]
- De March, N.; Prado, S.D.; Brunnet, L.G. Transport threshold in a quantum model for the Ksc, A ion channel. J. Phys. Condens. Matter. 2021, 34, 025101. [Google Scholar] [CrossRef]
- Serway, R.A.; Moses, C.J.; Moyer, C.A. Modern Physics; Cengage Learning: Boston, MA, USA, 2004. [Google Scholar]
- Chen, F.; Hihath, J.; Huang, Z.; Li, X.; Tao, N. Measurement of Single-Molecule Conductance. Annu. Rev. Phys. Chem. 2007, 58, 535–564. [Google Scholar] [CrossRef] [Green Version]
- Bertil, H.; Bertil, H. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textbook of Medical Physiology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kurbel, S.; Borzan, V.; Golem, H.; Dinjar, K. Cochlear potential difference between endolymph fluid and the hair cell’s interior: A retold interpretation based on the Goldman equation. Med. Glasnik. 2017, 14, 8–15. [Google Scholar]
- Qaswal, A.B. Magnesium Ions Depolarize the Neuronal Membrane via Quantum Tunneling through the Closed Channels. Quantum Rep. 2020, 2, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Waxman, S.G. Membranes, Myelin, and the Pathophysiology of Multiple Sclerosis. N. Engl. J. Med. 1982, 306, 1529–1533. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Sun, W.; Fu, Y.; Li, J.; Cheng, J.-X.; Nauman, E.; Shi, R. Compression Induces Acute Demyelination and Potassium Channel Exposure in Spinal Cord. J. Neurotrauma 2010, 27, 1109–1120. [Google Scholar] [CrossRef] [Green Version]
- Jukkola, P.I.; Lovett-Racke, A.E.; Zamvil, S.S.; Gu, C. K+ channel alterations in the progression of experimental autoimmune en-cephalomyelitis. Neurobiol. Dis. 2012, 47, 280–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, R.; Sun, W. Potassium channel blockers as an effective treatment to restore impulse conduction in injured axons. Neurosci. Bull. 2011, 27, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, R.; O’donnell, J.; Ding, F.; Nedergaard, M. Interstitial ions: A key regulator of state-dependent neural activity? Prog. Neurobiol. 2020, 193, 101802. [Google Scholar] [CrossRef]
- Taniguchi, M.; Yamada, Y.; Fukumoto, Y.; Sawano, S.; Minami, S.; Ikezoe, T.; Watanabe, Y.; Kimura, M.; Ichihashi, N. Increase in echo intensity and extracellular-to-intracellular water ratio is independently associated with muscle weakness in elderly women. Eur. J. Appl. Physiol. 2017, 117, 2001–2007. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.E. Voltage-Gated Channel Mechanosensitivity: Fact or Friction? Front. Physiol. 2011, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.A.; Lin, W.; Morris, T.; Banderali, U.; Juranka, P.F.; Morris, C.E. Membrane trauma and Na+leak from Nav1.6 channels. Am. J. Physiol. Physiol. 2009, 297, C823–C834. [Google Scholar] [CrossRef] [Green Version]
- Beyder, A.; Rae, J.L.; Bernard, C.; Strege, P.R.; Sachs, F.; Farrugia, G. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J. Physiol. 2010, 588, 4969–4985. [Google Scholar] [CrossRef]
- Qaswal, A.B. The Role of Quantum Tunneling of Ions in the Pathogenesis of the Cardiac Arrhythmias Due to Channelopa-thies, Ischemia, and Mechanical Stretch. Biophysics 2021, 66, 637–641. [Google Scholar] [CrossRef]
- Lee, S.; Briklin, O.; Hiel, H.; Fuchs, P. Calcium-dependent inactivation of calcium channels in cochlear hair cells of the chicken. J. Physiol. 2007, 583, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Chen, Y. Noise-Induced Hearing Loss: Updates on Molecular Targets and Potential Interventions. Neural Plast. 2021, 2021, 4784385. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J. Physiological mechanisms and neural models. In Tinnitus Handbook (Singular Audiology Text); Singular Pub. Group: San Diego, CA, USA, 2000; pp. 85–122. [Google Scholar]
- Kelley, R.E. Ischemic demyelination. Neurol. Res. 2006, 28, 334–340. [Google Scholar] [CrossRef]
- Nukada, H.; Dyck, P.J. Acute ischemia causes axonal stasis, swelling, attenuation, and secondary demyelination. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1987, 22, 311–318. [Google Scholar] [CrossRef]
- Sharma, K.R.; Cross, J.; Farronay, O.; Ayyar, D.R.; Shebert, R.T.; Bradley, W.G. Demyelinating Neuropathy in Diabetes Mellitus. Arch. Neurol. 2002, 59, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Peterson, L.K.; Fujinami, R.S. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of mul-tiple sclerosis. J. Neuroimmunol. 2007, 184, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Faber, D.S.; Pereda, A.E. Two Forms of Electrical Transmission Between Neurons. Front. Mol. Neurosci. 2018, 11, 427. [Google Scholar] [CrossRef]
- Sindhusake, D.; Golding, M.; Wigney, D.; Newall, P.; Jakobsen, K.; Mitchell, P. Factors predicting severity of tinnitus: A popula-tion-based assessment. J. Am. Acad. Audiol. 2004, 15, 269–280. [Google Scholar]
- Soler-Llavina, G.J.; Holmgren, M.; Swartz, K.J. Defining the Conductance of the Closed State in a Voltage-Gated K+ Channel. Neuron 2003, 38, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Banh, R.; Cherny, V.V.; Morgan, D.; Musset, B.; Thomas, S.; Kulleperuma, K.; Smith, S.M.; Pomès, R.; De Coursey, T.E. Hydrophobic gas-ket mutation produces gating pore currents in closed human voltage-gated proton channels. Proc. Natl. Acad. Sci. USA 2019, 116, 18951–18961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastassiou, C.A.; Perin, R.; Markram, H.; Koch, C. Ephaptic coupling of cortical neurons. Nat. Neurosci. 2011, 14, 217–223. [Google Scholar] [CrossRef] [PubMed]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rawashdeh, B.M.; Qaswal, A.B.; Suleiman, A.; Zayed, F.M.; Al-Rawashdeh, S.M.; Tawalbeh, M.; Khreesha, L.; Alzubaidi, A.; Al-Zubidi, E.; Ghala, Z.; et al. The Quantum Tunneling of Ions Model Can Explain the Pathophysiology of Tinnitus. Brain Sci. 2022, 12, 426. https://doi.org/10.3390/brainsci12040426
Al-Rawashdeh BM, Qaswal AB, Suleiman A, Zayed FM, Al-Rawashdeh SM, Tawalbeh M, Khreesha L, Alzubaidi A, Al-Zubidi E, Ghala Z, et al. The Quantum Tunneling of Ions Model Can Explain the Pathophysiology of Tinnitus. Brain Sciences. 2022; 12(4):426. https://doi.org/10.3390/brainsci12040426
Chicago/Turabian StyleAl-Rawashdeh, Baeth M, Abdallah Barjas Qaswal, Aiman Suleiman, Fuad Mohammed Zayed, S. M. Al-Rawashdeh, Mohamed Tawalbeh, Lubna Khreesha, Ayham Alzubaidi, Enas Al-Zubidi, Zuhir Ghala, and et al. 2022. "The Quantum Tunneling of Ions Model Can Explain the Pathophysiology of Tinnitus" Brain Sciences 12, no. 4: 426. https://doi.org/10.3390/brainsci12040426