Deciding on Optical Illusions: Reduced Alpha Power in Body Dysmorphic Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
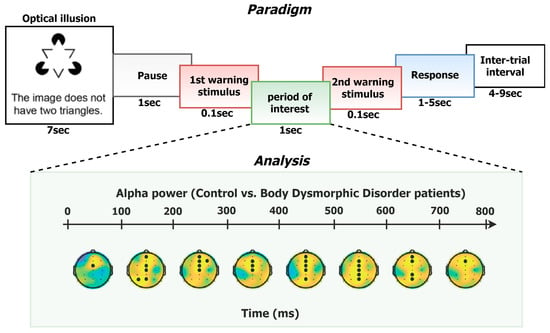
2.2. Experimental Design and Procedure
2.3. EEG Recording and Preprocessing
2.4. Psychometric Ratings
- Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) for BDD: This psychometric questionnaire evaluates the severity of BDD symptoms [34]. We used a 12-item version translated, adapted and validated in Greek [35]. Items 1–5 assess obsessional preoccupation with the perceived defect in appearance, while items 6–10 assess compulsive behaviors. Item 11 measures the degree of insight, and item 12 avoidance. It is rated on a 0 (not at all) to 4 (every day) Likert scale. Scores for all items are summed up to create the total score.
- Dysmorphic Concern Questionnaire (DCQ): This questionnaire is a 7-item self-report measure that assesses cognitive and behavioral symptoms of physical overconcern without seeking to establish a “diagnosis” of BDD [36]. Respondents rate their concern on their physical appearance on a 4-point scale, ranging from 0 (not at all) to 3 (much more than most people).
2.5. Data Analysis
2.5.1. Behavioral Analysis
2.5.2. EEG Analysis
- (1)
- i and j belongs to neighboring electrodes
- (2)
- i and j belongs to successive time-points
- (3)
- i and j have the same sign.
2.5.3. Relationship between EEG Measures and BDD Severity
3. Results
3.1. Behavioral Results
3.2. EEG Results
3.3. Relationship between EEG Measures and BDD Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buhlmann, U.; McNally, R.J.; Wilhelm, S.; Florin, I. Selective processing of emotional information in body dysmorphic disorder. J. Anxiety Disord. 2002, 16, 289–298. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5 (R.)); Psychiatric Association Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Bruno, N. Unifying sequential effects in perceptual grouping. Trends Cogn. Sci. 2005, 9, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sterzer, P.; Rees, G. A neural basis for percept stabilization in binocular rivalry. J. Cogn. Neurosci. 2008, 20, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Herrmann, C.S. Illusory contours: A window onto the neurophysiology of constructing perception. Trends Cogn. Sci. 2013, 17, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, C.; Stachtea, X.; Papageorgiou, P.; Alexandridis, A.T.; Makris, G.; Chrousos, G.; Kosteletos, G. Gender-dependent variations in optical illusions: Evidence from N400 waveforms. Physiol. Meas. 2020, 41, 095006. [Google Scholar] [CrossRef]
- Gregory, R.L. Visual illusions classified. Trends Cogn. Sci. 1997, 1, 190–194. [Google Scholar] [CrossRef]
- Eagleman, D.M. Visual illusions and neurobiology. Nat. Rev. Neurosci. 2001, 2, 920–926. [Google Scholar] [CrossRef] [PubMed]
- King, D.J.; Hodgekins, J.; Chouinard, P.A.; Chouinard, V.-A.; Sperandio, I. A review of abnormalities in the perception of visual illusions in schizophrenia. Psychon. Bull. Rev. 2017, 24, 734–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toh, W.L.; Castle, D.J.; Rossell, S. Examining neurocognition in body dysmorphic disorder using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): A comparison with obsessive-compulsive disorder. Psychiatry Res. 2015, 228, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Feusner, J.D.; Moller, H.; Altstein, L.; Sugar, C.; Bookheimer, S.; Yoon, J.; Hembacher, E. Inverted face processing in body dysmorphic disorder. J. Psychiatr. Res. 2010, 44, 1088–1094. [Google Scholar] [CrossRef] [Green Version]
- Stangier, U.; Adam-Schwebe, S.; Müller, T.; Wolter, M. Discrimination of facial appearance stimuli in body dysmorphic disorder. J. Abnorm. Psychol. 2008, 117, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Yaryura-Tobias, J.A.; Neziroglu, F.; Chang, R.; Lee, S.; Pinto, A.; Donohue, L. Computerized Perceptual Analysis of Patients with Body Dysmorphic Disorder: A Pilot Study. CNS Spectr. 2002, 7, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Buhlmann, U.; McNally, R.J.; Etcoff, N.L.; Tuschen-Caffier, B.; Wilhelm, S. Emotion recognition deficits in body dysmorphic disorder. J. Psychiatr. Res. 2004, 38, 201–206. [Google Scholar] [CrossRef]
- Toh, W.L.; Castle, D.J.; Rossell, S.L. Attentional biases in body dysmorphic disorder (BDD): Eye-tracking using the emotional Stroop task. Compr. Psychiatry 2017, 74, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Radomsky, A.; Rachman, S.; Hammond, D. Memory bias, confidence and responsibility in compulsive checking. Behav. Res. Ther. 2001, 39, 813–822. [Google Scholar] [CrossRef]
- van den Hout, M.; Kindt, M. Obsessive–compulsive disorder and the paradoxical effects of perseverative behaviour on experienced uncertainty. J. Behav. Ther. Exp. Psychiatry 2004, 35, 165–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolin, D.F.; Abramowitz, J.S.; Brigidi, B.D.; Amir, N.; Street, G.P.; Foa, E.B. Memory and memory confidence in obsessive–compulsive disorder. Behav. Res. Ther. 2001, 39, 913–927. [Google Scholar] [CrossRef]
- Kapsali, F.; Zioga, I.; Papageorgiou, P.; Smyrnis, N.; Chrousos, G.P.; Papageorgiou, C. Event-related EEG oscillations in body dysmorphic disorder. Eur. J. Clin. Investig. 2020, 50, e13208. [Google Scholar] [CrossRef]
- Min, B.-K.; Kim, S.J.; Park, J.Y.; Park, H.-J. Prestimulus top-down reflection of obsessive-compulsive disorder in EEG frontal theta and occipital alpha oscillations. Neurosci. Lett. 2011, 496, 181–185. [Google Scholar] [CrossRef]
- Perera, M.P.N.; Bailey, N.W.; Herring, S.E.; Fitzgerald, P.B. Electrophysiology of obsessive compulsive disorder: A systematic review of the electroencephalographic literature. J. Anxiety Disord. 2019, 62, 1–14. [Google Scholar] [CrossRef]
- Simpson, H.B.; Tenke, C.; Towey, J.B.; Liebowitz, M.R.; Bruder, G.E. Symptom provocation alters behavioral ratings and brain electrical activity in obsessive–compulsive disorder: A preliminary study. Psychiatry Res. 2000, 95, 149–155. [Google Scholar] [CrossRef]
- Giannopoulos, A.E.; Zioga, I.; Papageorgiou, P.C.; Kapsali, F.; Spantideas, S.T.; Kapsalis, N.C.; Capsalis, C.N.; Kontoangelos, K.; Papageorgiou, C.C. Early auditory-evoked potentials in body dysmorphic disorder: An ERP/sLORETA study. Psychiatry Res. 2021, 299, 113865. [Google Scholar] [CrossRef]
- Mataix-Cols, D.; Wooderson, S.; Lawrence, N.; Brammer, M.J.; Speckens, A.; Phillips, M.L. Distinct Neural Correlates of Washing, Checking, and Hoarding SymptomDimensions in Obsessive-compulsive Disorder. Arch. Gen. Psychiatry 2004, 61, 564–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, O.; Mazaheri, A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [Green Version]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Palva, S.; Palva, J.M. New vistas for α-frequency band oscillations. Trends Neurosci. 2007, 30, 150–158. [Google Scholar] [CrossRef]
- Nedeljkovic, M.; Moulding, R.; Kyrios, M.; Doron, G. The relationship of cognitive confidence to OCD symptoms. J. Anxiety Disord. 2009, 23, 463–468. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Chaumon, M.; Bishop, D.V.; Busch, N.A. A practical guide to the selection of independent components of the electroencephalogram for artifact correction. J. Neurosci. Methods 2015, 250, 47–63. [Google Scholar] [CrossRef]
- Winkler, I.; Haufe, S.; Tangermann, M. Automatic Classification of Artifactual ICA-Components for Artifact Removal in EEG Signals. Behav. Brain Funct. 2011, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Mognon, A.; Jovicich, J.; Bruzzone, L.; Buiatti, M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology 2011, 48, 229–240. [Google Scholar] [CrossRef]
- Nolan, H.; Whelan, R.; Reilly, R. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. J. Neurosci. Methods 2010, 192, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.A.; Hollander, E.; Rasmussen, S.A.; Aronowitz, B.R.; Decaria, C.; Goodman, W.K. A severity rating scale for body dysmorphic disorder: Development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol. Bull. 1997, 33, 17. [Google Scholar] [PubMed]
- Kapsali, F.; Nikolaou, P.; Papageorgiou, C. Yale-Brown Obsessive Compulsive Scale Modified for Body Dysmorphic Disorder (BDD-YBOCS): Greek Translation, Validation and Psychometric Properties. EC Psychol. Psychiatry 2019, 8, 884–894. [Google Scholar]
- Oosthuizen, P.; Lambert, T.; Castle, D.J. Dysmorphic concern: Prevalence and associations with clinical variables. Aust. N. Z. J. Psychiatry 1998, 32, 129–132. [Google Scholar] [CrossRef]
- Monzani, B.; Krebs, G.; Anson, M.; Veale, D.; Mataix-Cols, D. Holistic versus detailed visual processing in body dysmorphic disorder: Testing the inversion, composite and global precedence effects. Psychiatry Res. 2013, 210, 994–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.; Williamson, P.; Wade, T.D. A systematic review and meta-analysis of cognitive processing deficits associated with body dysmorphic disorder. Behav. Res. Ther. 2018, 107, 83–94. [Google Scholar] [CrossRef]
- Hermans, D.; Engelen, U.; Grouwels, L.; Joos, E.; Lemmens, J.; Pieters, G. Cognitive confidence in obsessive-compulsive disorder: Distrusting perception, attention and memory. Behav. Res. Ther. 2008, 46, 98–113. [Google Scholar] [CrossRef]
- Otto, M.W. Normal and abnormal information processing: A neuropsychological perspective on obsessive compulsive disorder. Psychiatr. Clin. N. Am. 1992, 15, 825–848. [Google Scholar] [CrossRef]
- Foxe, J.J.; Snyder, A.C. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef] [Green Version]
- Crawford, H.J.; Knebel, T.L.; Vendemia, J.M.; Kaplan, L.; Ratcliff, B. EEG activation patterns during tracking and decision-making tasks—Differences between low and high sustained attention adults. In Proceedings of the 8th International Symposium on Aviation Psychology, Columbus, OH, USA, 24–27 April 1995; pp. 886–890. [Google Scholar]
- Haegens, S.; Händel, B.F.; Jensen, O. Top-Down Controlled Alpha Band Activity in Somatosensory Areas Determines Behavioral Performance in a Discrimination Task. J. Neurosci. 2011, 31, 5197–5204. [Google Scholar] [CrossRef] [PubMed]
- Pogarell, O.; Juckel, G.; Mavrogiorgou, P.; Mulert, C.; Folkerts, M.; Hauke, W.; Zaudig, M.; Möller, H.-J.; Hegerl, U. Symptom-specific EEG power correlations in patients with obsessive–compulsive disorder. Int. J. Psychophysiol. 2006, 62, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Keil, J.; Schnitzler, A.; van Dijk, H.; Weisz, N. The role of alpha oscillations for illusory perception. Behav. Brain Res. 2014, 271, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Bucci, P.; Mucci, A.; Volpe, U.; Merlotti, E.; Galderisi, S.; Maj, M. Executive hypercontrol in obsessive–compulsive disorder: Electrophysiological and neuropsychological indices. Clin. Neurophysiol. 2004, 115, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, A.E.; Spantideas, S.T.; Capsalis, C.; Papageorgiou, P.; Kapsalis, N.; Kontoangelos, K.; Papageorgiou, C. Instantaneous radiated power of brain activity: Application to prepulse inhibition and facilitation for body dysmorphic disorder. Biomed. Eng. Online 2021, 20, 1–21. [Google Scholar] [CrossRef]
- Feusner, J.D.; Townsend, J.; Bystritsky, A.; Bookheimer, S. Visual Information Processing of Faces in Body Dysmorphic Disorder. Arch. Gen. Psychiatry 2007, 64, 1417–1425. [Google Scholar] [CrossRef] [Green Version]
- Feusner, J.D.; Moody, T.; Hembacher, E.; Townsend, J.; McKinley, M.; Moller, H.; Bookheimer, S. Abnormalities of Visual Processing and Frontostriatal Systems in Body Dysmorphic Disorder. Arch. Gen. Psychiatry 2010, 67, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Scholz, S.; Schneider, S.L.; Rose, M. Differential effects of ongoing EEG beta and theta power on memory formation. PLoS ONE 2017, 12, e0171913. [Google Scholar] [CrossRef]
- Feusner, J.D.; Townsend, J.; Bystritsky, A.; McKinley, M.; Moller, H.; Bookheimer, S. Regional brain volumes and symptom severity in body dysmorphic disorder. Psychiatry Res. Neuroimaging 2009, 172, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannopoulos, A.E.; Zioga, I.; Kontoangelos, K.; Papageorgiou, P.; Kapsali, F.; Capsalis, C.N.; Papageorgiou, C. Deciding on Optical Illusions: Reduced Alpha Power in Body Dysmorphic Disorder. Brain Sci. 2022, 12, 293. https://doi.org/10.3390/brainsci12020293
Giannopoulos AE, Zioga I, Kontoangelos K, Papageorgiou P, Kapsali F, Capsalis CN, Papageorgiou C. Deciding on Optical Illusions: Reduced Alpha Power in Body Dysmorphic Disorder. Brain Sciences. 2022; 12(2):293. https://doi.org/10.3390/brainsci12020293
Chicago/Turabian StyleGiannopoulos, Anastasios E., Ioanna Zioga, Konstantinos Kontoangelos, Panos Papageorgiou, Fotini Kapsali, Christos N. Capsalis, and Charalabos Papageorgiou. 2022. "Deciding on Optical Illusions: Reduced Alpha Power in Body Dysmorphic Disorder" Brain Sciences 12, no. 2: 293. https://doi.org/10.3390/brainsci12020293