Targeting NMDA Receptors in Emotional Disorders: Their Role in Neuroprotection
Abstract
:1. Introduction
2. NMDA Receptors
3. NMDAR-Mediated Signaling in Brain Functioning
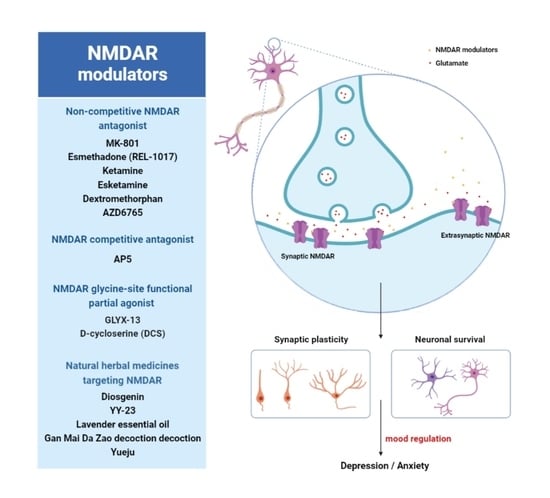
3.1. Synaptic Plasticity
3.2. Neuronal Survival
4. Role of NMDA Receptors in Anxiety
| Compound | Mechanism of Action | Effect |
|---|---|---|
| D-cycloserine (DCS) | NMDAR glycine partial agonist | Augmenting extinction learning [93]; adjunct treatment to exposure therapy for anxiety disorders [82] |
| Ketamine | Non-competitive NMDA channel blocker | Reducing anxiety in treatment-resistant generalized anxiety and social anxiety disorders [86,94] |
| Memantine | Non-competitive NMDAR antagonist | Treatment-resistant obsessive-compulsive disorder [95] |
| Amantadine | NMDAR antagonist | Augmentation therapy for obsessive-compulsive patients resistant to SSRIs [96] |
5. Role of NMDA Receptors in Depression
| Compound | Mechanism of Action | Effect |
|---|---|---|
| REL-1017 (Esmethadone) | NMDAR channel blocker | Adjunctive treatment in MDD [127] |
| Ketamine | Non-competitive NMDA channel blocker | Antidepressant mechanisms in TRD and SSRI-resistant depression [121,138,139] |
| Esketamine | Non-competitive NMDA channel blocker | Antidepressant mechanisms in TRD [15] |
| Dextro-methorphan | Non-competitive NMDAR antagonist | Novel antidepressant in treatment-resistant MDD [140] |
| AZD6765 | Low-trapping NMDA antagonist | Rapid antidepressant effects in MDD [128] |
| Nitrous oxide | NMDAR antagonist | Adjunctive therapy in MDD [141] |
| D-cycloserine (DCS) | NMDAR glycine partial agonist | Adjuvant therapy for treatment-resistant MDD [130,142] |
| L-4-chlorokynurenine (4-Cl-KYN) | glycine site NMDAR antagonist | Rapid antidepressant effects in TRD [143] |
| GLYX-13 | NMDAR glycine site functional partial agonist | Antidepressant treatment in MDD [131,133] |
| Traxoprodil (CP-101,606) | NR2B subunit-selective NMDAR antagonist | Antidepressant effects in treatment-resistant MDD [144] |
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fullana, M.A.; Hidalgo-Mazzei, D.; Vieta, E.; Radua, J. Coping behaviors associated with decreased anxiety and depressive symptoms during the COVID-19 pandemic and lockdown. J. Affect. Disord. 2020, 275, 80–81. [Google Scholar] [CrossRef]
- Hyland, P.; Shevlin, M.; McBride, O.; Murphy, J.; Karatzias, T.; Bentall, R.P.; Martinez, A.; Vallières, F. Anxiety and depression in the Republic of Ireland during the COVID-19 pandemic. Acta Psychiatr. Scand. 2020, 142, 249–256. [Google Scholar] [CrossRef]
- Lei, L.; Huang, X.; Zhang, S.; Yang, J.; Yang, L.; Xu, M. Comparison of Prevalence and Associated Factors of Anxiety and Depression Among People Affected by versus People Unaffected by Quarantine During the COVID-19 Epidemic in Southwestern China. Med. Sci. Monit. 2020, 26, e924609. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liao, Z.; Huang, H.; Jiang, B.; Zhang, X.; Wang, Y.; Zhao, M. Comparison of the Indicators of Psychological Stress in the Population of Hubei Province and Non-Endemic Provinces in China During Two Weeks During the Coronavirus Disease 2019 (COVID-19) Outbreak in February 2020. Med. Sci. Monit. 2020, 26, e923767. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Hosseinian-Far, A.; Jalali, R.; Vaisi-Raygani, A.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Glob. Health 2020, 16, 57. [Google Scholar] [CrossRef]
- Moreno, C.; Wykes, T.; Galderisi, S.; Nordentoft, M.; Crossley, N.; Jones, N.; Cannon, M.; Correll, C.U.; Byrne, L.; Carr, S.; et al. How mental health care should change as a consequence of the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 813–824. [Google Scholar] [CrossRef]
- Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef]
- Bertolote, J.M.; Fleischmann, A.; De Leo, D.; Wasserman, D. Psychiatric diagnoses and suicide: Revisiting the evidence. Crisis 2004, 25, 147–155. [Google Scholar] [CrossRef]
- Kohn, R.; Saxena, S.; Levav, I.; Saraceno, B. The treatment gap in mental health care. Bull. World Health Organ. 2004, 82, 858–866. [Google Scholar] [PubMed]
- Barnes, T.R.; Drake, R.; Paton, C.; Cooper, S.J.; Deakin, B.; Ferrier, I.N.; Gregory, C.J.; Haddad, P.M.; Howes, O.D.; Jones, I.; et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: Updated recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2020, 34, 3–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumberg, M.J.; Vaccarino, S.R.; McInerney, S.J. Procognitive Effects of Antidepressants and Other Therapeutic Agents in Major Depressive Disorder: A Systematic Review. J. Clin. Psychiatry 2020, 81, 15692. [Google Scholar] [CrossRef]
- McGrath, T.; Baskerville, R.; Rogero, M.; Castell, L. Emerging Evidence for the Widespread Role of Glutamatergic Dysfunction in Neuropsychiatric Diseases. Nutrients 2022, 14, 917. [Google Scholar] [CrossRef] [PubMed]
- Li, C.T.; Yang, K.C.; Lin, W.C. Glutamatergic Dysfunction and Glutamatergic Compounds for Major Psychiatric Disorders: Evidence From Clinical Neuroimaging Studies. Front. Psychiatry 2018, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Farchione, T.; Potter, A.; Chen, Q.; Temple, R. Esketamine for Treatment-Resistant Depression-First FDA-Approved Antidepressant in a New Class. N. Engl. J. Med. 2019, 381, 1–4. [Google Scholar] [CrossRef]
- Manji, H.K.; Moore, G.J.; Rajkowska, G.; Chen, G. Neuroplasticity and cellular resilience in mood disorders. Mol. Psychiatry 2000, 5, 578–593. [Google Scholar] [CrossRef]
- Malykhin, N.V.; Coupland, N.J. Hippocampal neuroplasticity in major depressive disorder. Neuroscience 2015, 309, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar]
- Paoletti, P. Molecular basis of NMDA receptor functional diversity. Eur. J. Neurosci. 2011, 33, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Dunah, A.W.; Standaert, D.G. Subcellular segregation of distinct heteromeric NMDA glutamate receptors in the striatum. J. Neurochem. 2003, 85, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Brothwell, S.L.; Barber, J.L.; Monaghan, D.T.; Jane, D.E.; Gibb, A.J.; Jones, S. NR2B- and NR2D-containing synaptic NMDA receptors in developing rat substantia nigra pars compacta dopaminergic neurones. J. Physiol. 2008, 586, 739–750. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Yong, X.L.H.; Roche, K.W.; Anggono, V. Regulation of NMDA glutamate receptor functions by the GluN2 subunits. J. Neurochem. 2020, 154, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Fetterolf, F.; Foster, K.A. Regulation of long-term plasticity induction by the channel and C-terminal domains of GluN2 subunits. Mol. Neurobiol. 2011, 44, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, L.A.; Bernardinelli, Y.; Muller, D. GluN3A: An NMDA receptor subunit with exquisite properties and functions. Neural Plast 2013, 2013, 145387. [Google Scholar] [CrossRef]
- Wong, H.K.; Liu, X.B.; Matos, M.F.; Chan, S.F.; Pérez-Otaño, I.; Boysen, M.; Cui, J.; Nakanishi, N.; Trimmer, J.S.; Jones, E.G.; et al. Temporal and regional expression of NMDA receptor subunit NR3A in the mammalian brain. J. Comp. Neurol. 2002, 450, 303–317. [Google Scholar] [CrossRef]
- Matsuda, K.; Kamiya, Y.; Matsuda, S.; Yuzaki, M. Cloning and characterization of a novel NMDA receptor subunit NR3B: A dominant subunit that reduces calcium permeability. Brain Res. Mol. Brain Res. 2002, 100, 43–52. [Google Scholar] [CrossRef]
- Elmasri, M.; Hunter, D.W.; Winchester, G.; Bates, E.E.; Aziz, W.; Van Der Does, D.M.; Karachaliou, E.; Sakimura, K.; Penn, A.C. Common synaptic phenotypes arising from diverse mutations in the human NMDA receptor subunit GluN2A. Commun. Biol. 2022, 5, 174. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, M.; Lotti, J.S.; Aziz, W.; Steele, O.G.; Karachaliou, E.; Sakimura, K.; Hansen, K.B.; Penn, A.C. Synaptic Dysfunction by Mutations in GRIN2B: Influence of Triheteromeric NMDA Receptors on Gain-of-Function and Loss-of-Function Mutant Classification. Brain Sci. 2022, 12, 789. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.S.; Spitzer, N.C. Calcium signaling in neuronal development. Cold Spring Harb. Perspect. Biol. 2011, 3, a004259. [Google Scholar] [CrossRef]
- Malenka, R.C.; Nicoll, R.A. NMDA-receptor-dependent synaptic plasticity: Multiple forms and mechanisms. Trends Neurosci. 1993, 16, 521–527. [Google Scholar] [CrossRef]
- Pagano, J.; Giona, F.; Beretta, S.; Verpelli, C.; Sala, C. N-methyl-d-aspartate receptor function in neuronal and synaptic development and signaling. Curr. Opin. Pharmacol. 2021, 56, 93–101. [Google Scholar] [CrossRef]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef]
- Lan, J.Y.; Skeberdis, V.A.; Jover, T.; Grooms, S.Y.; Lin, Y.; Araneda, R.C.; Zheng, X.; Bennett, M.V.; Zukin, R.S. Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 2001, 4, 382–390. [Google Scholar] [CrossRef]
- Grosshans, D.R.; Clayton, D.A.; Coultrap, S.J.; Browning, M.D. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat. Neurosci. 2002, 5, 27–33. [Google Scholar] [CrossRef]
- Lin, Y.; Jover-Mengual, T.; Wong, J.; Bennett, M.V.; Zukin, R.S. PSD-95 and PKC converge in regulating NMDA receptor trafficking and gating. Proc. Natl. Acad. Sci. USA 2006, 103, 19902–19907. [Google Scholar] [CrossRef]
- Murphy, J.A.; Stein, I.S.; Lau, C.G.; Peixoto, R.T.; Aman, T.K.; Kaneko, N.; Aromolaran, K.; Saulnier, J.L.; Popescu, G.K.; Sabatini, B.L.; et al. Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines. J. Neurosci. 2014, 34, 869–879. [Google Scholar] [CrossRef]
- Skeberdis, V.A.; Chevaleyre, V.; Lau, C.G.; Goldberg, J.H.; Pettit, D.L.; Suadicani, S.O.; Lin, Y.; Bennett, M.V.; Yuste, R.; Castillo, P.E.; et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nat. Neurosci. 2006, 9, 501–510. [Google Scholar] [CrossRef]
- Lüscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaac, J. Protein phosphatase 1 and LTD: Synapses are the architects of depression. Neuron 2001, 32, 963–966. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Peineau, S.; Howland, J.G.; Wang, Y.T. Long-term depression in the CNS. Nat. Rev. Neurosci. 2010, 11, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.; Kessels, H.W.; Alfonso, S.; Aow, J.; Fox, R.; Malinow, R. Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc. Natl. Acad. Sci. USA 2013, 110, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Stein, I.S.; Gray, J.A.; Zito, K. Non-Ionotropic NMDA Receptor Signaling Drives Activity-Induced Dendritic Spine Shrinkage. J. Neurosci. 2015, 35, 12303–12308. [Google Scholar] [CrossRef] [PubMed]
- Stein, I.S.; Park, D.K.; Flores, J.C.; Jahncke, J.N.; Zito, K. Molecular Mechanisms of Non-ionotropic NMDA Receptor Signaling in Dendritic Spine Shrinkage. J. Neurosci. 2020, 40, 3741–3750. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Q.H.; Duan, K.; Li, Z. NMDA receptor-dependent LTD is required for consolidation but not acquisition of fear memory. J. Neurosci. 2014, 34, 8741–8748. [Google Scholar] [CrossRef]
- Volianskis, A.; France, G.; Jensen, M.S.; Bortolotto, Z.A.; Jane, D.E.; Collingridge, G.L. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 2015, 1621, 5–16. [Google Scholar] [CrossRef]
- Volianskis, A.; Bannister, N.; Collett, V.J.; Irvine, M.W.; Monaghan, D.T.; Fitzjohn, S.M.; Jensen, M.S.; Jane, D.E.; Collingridge, G.L. Different NMDA receptor subtypes mediate induction of long-term potentiation and two forms of short-term potentiation at CA1 synapses in rat hippocampus in vitro. J. Physiol. 2013, 591, 955–972. [Google Scholar] [CrossRef]
- Wong, J.M.; Gray, J.A. Long-Term Depression Is Independent of GluN2 Subunit Composition. J. Neurosci. 2018, 38, 4462–4470. [Google Scholar] [CrossRef]
- Hardingham, G.E. Pro-survival signalling from the NMDA receptor. Biochem. Soc. Trans. 2006, 34, 936–938. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Motaghinejad, M.; Motevalian, M.; Fatima, S.; Beiranvand, T.; Mozaffari, S. Topiramate via NMDA, AMPA/kainate, GABA(A) and Alpha2 receptors and by modulation of CREB/BDNF and Akt/GSK3 signaling pathway exerts neuroprotective effects against methylphenidate-induced neurotoxicity in rats. J. Neural. Transm. 2017, 124, 1369–1387. [Google Scholar] [CrossRef]
- Shin, M.K.; Jung, W.R.; Kim, H.G.; Roh, S.E.; Kwak, C.H.; Kim, C.H.; Kim, S.J.; Kim, K.L. The ganglioside GQ1b regulates BDNF expression via the NMDA receptor signaling pathway. Neuropharmacology 2014, 77, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Buchthal, B.; Lau, D.; Hayer, S.; Dick, O.; Schwaninger, M.; Veltkamp, R.; Zou, M.; Weiss, U.; Bading, H. A signaling cascade of nuclear calcium-CREB-ATF3 activated by synaptic NMDA receptors defines a gene repression module that protects against extrasynaptic NMDA receptor-induced neuronal cell death and ischemic brain damage. J. Neurosci. 2011, 31, 4978–4990. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Lason, W. Different mechanisms of NMDA-mediated protection against neuronal apoptosis: A stimuli-dependent effect. Neurochem. Res. 2009, 34, 2040–2054. [Google Scholar] [CrossRef]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef]
- Dick, O.; Bading, H. Synaptic activity and nuclear calcium signaling protect hippocampal neurons from death signal-associated nuclear translocation of FoxO3a induced by extrasynaptic N-methyl-D-aspartate receptors. J. Biol. Chem. 2010, 285, 19354–19361. [Google Scholar] [CrossRef]
- Papadia, S.; Soriano, F.X.; Léveillé, F.; Martel, M.A.; Dakin, K.A.; Hansen, H.H.; Kaindl, A.; Sifringer, M.; Fowler, J.; Stefovska, V.; et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 2008, 11, 476–487. [Google Scholar] [CrossRef]
- Sala, C.; Rudolph-Correia, S.; Sheng, M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J. Neurosci. 2000, 20, 3529–3536. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Fukunaga, Y.; Bading, H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002, 5, 405–414. [Google Scholar] [CrossRef]
- Ivanov, A.; Pellegrino, C.; Rama, S.; Dumalska, I.; Salyha, Y.; Ben-Ari, Y.; Medina, I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J. Physiol. 2006, 572, 789–798. [Google Scholar] [CrossRef]
- Puddifoot, C.; Martel, M.A.; Soriano, F.X.; Camacho, A.; Vidal-Puig, A.; Wyllie, D.J.; Hardingham, G.E. PGC-1α negatively regulates extrasynaptic NMDAR activity and excitotoxicity. J. Neurosci. 2012, 32, 6995–7000. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, Q.; Zhang, M.; Wang, H.; Yun, W.; Zhou, X. Co-activation of synaptic and extrasynaptic NMDA receptors by neuronal insults determines cell death in acute brain slice. Neurochem. Int. 2014, 78, 28–34. [Google Scholar] [CrossRef]
- Zhou, X.; Hollern, D.; Liao, J.; Andrechek, E.; Wang, H. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell. Death Dis. 2013, 4, e560. [Google Scholar] [CrossRef]
- Victoriano, G.; Santos-Costa, N.; Mascarenhas, D.C.; Nunes-de-Souza, R.L. Inhibition of the left medial prefrontal cortex (mPFC) prolongs the social defeat-induced anxiogenesis in mice: Attenuation by NMDA receptor blockade in the right mPFC. Behav. Brain Res. 2020, 378, 112312. [Google Scholar] [CrossRef]
- Walker, D.L.; Davis, M. Role of the extended amygdala in short-duration versus sustained fear: A tribute to Dr. Lennart Heimer. Brain Struct. Funct. 2008, 213, 29–42. [Google Scholar] [CrossRef]
- Lehner, M.; Wisłowska-Stanek, A.; Taracha, E.; Maciejak, P.; Szyndler, J.; Skórzewska, A.; Turzyńska, D.; Sobolewska, A.; Hamed, A.; Bidziński, A.; et al. The expression of c-Fos and colocalisation of c-Fos and glucocorticoid receptors in brain structures of low and high anxiety rats subjected to extinction trials and re-learning of a conditioned fear response. Neurobiol. Learn. Mem. 2009, 92, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Lehner, M.; Taracha, E.; Skórzewska, A.; Turzyńska, D.; Sobolewska, A.; Maciejak, P.; Szyndler, J.; Hamed, A.; Bidziński, A.; Wisłowska-Stanek, A.; et al. Expression of c-Fos and CRF in the brains of rats differing in the strength of a fear response. Behav. Brain Res. 2008, 188, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Salimando, G.J.; Hyun, M.; Boyt, K.M.; Winder, D.G. BNST GluN2D-Containing NMDA Receptors Influence Anxiety- and Depressive-like Behaviors and ModulateCell-Specific Excitatory/Inhibitory Synaptic Balance. J. Neurosci. 2020, 40, 3949–3968. [Google Scholar] [CrossRef]
- Glangetas, C.; Massi, L.; Fois, G.R.; Jalabert, M.; Girard, D.; Diana, M.; Yonehara, K.; Roska, B.; Xu, C.; Lüthi, A.; et al. NMDA-receptor-dependent plasticity in the bed nucleus of the stria terminalis triggers long-term anxiolysis. Nat. Commun. 2017, 8, 14456. [Google Scholar] [CrossRef] [Green Version]
- Riaza Bermudo-Soriano, C.; Perez-Rodriguez, M.M.; Vaquero-Lorenzo, C.; Baca-Garcia, E. New perspectives in glutamate and anxiety. Pharmacol. Biochem. Behav. 2012, 100, 752–774. [Google Scholar] [CrossRef]
- Harvey, B.H.; Shahid, M. Metabotropic and ionotropic glutamate receptors as neurobiological targets in anxiety and stress-related disorders: Focus on pharmacology and preclinical translational models. Pharmacol. Biochem. Behav. 2012, 100, 775–800. [Google Scholar] [CrossRef]
- Zhan, Y.; Xia, J.; Wang, X. Effects of glutamate-related drugs on anxiety and compulsive behavior in rats with obsessive-compulsive disorder. Int. J. Neurosci. 2020, 130, 551–560. [Google Scholar] [CrossRef]
- Boyce-Rustay, J.M.; Holmes, A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology 2006, 31, 2405–2414. [Google Scholar] [CrossRef]
- Lehner, M.; Wisłowska-Stanek, A.; Gryz, M.; Sobolewska, A.; Turzyńska, D.; Chmielewska, N.; Krząścik, P.; Skórzewska, A.; Płaźnik, A. The co-expression of GluN2B subunits of the NMDA receptors and glucocorticoid receptors after chronic restraint stress in low and high anxiety rats. Behav. Brain Res. 2017, 319, 124–134. [Google Scholar] [CrossRef]
- De Souza Silva, M.A.; Marchetti, L.; Eisel, U.L.; Huston, J.P.; Dere, E. NR2C by NR2B subunit exchange in juvenile mice affects emotionality and 5-HT in the frontal cortex. Genes Brain Behav. 2007, 6, 465–472. [Google Scholar] [CrossRef]
- Du Bois, T.M.; Huang, X.F. Early brain development disruption from NMDA receptor hypofunction: Relevance to schizophrenia. Brain Res. Rev. 2007, 53, 260–270. [Google Scholar] [CrossRef]
- Amani, M.; Samadi, H.; Doosti, M.H.; Azarfarin, M.; Bakhtiari, A.; Majidi-Zolbanin, N.; Mirza-Rahimi, M.; Salari, A.A. Neonatal NMDA receptor blockade alters anxiety- and depression-related behaviors in a sex-dependent manner in mice. Neuropharmacology 2013, 73, 87–97. [Google Scholar] [CrossRef]
- Sun, H.; Jia, N.; Guan, L.; Su, Q.; Wang, D.; Li, H.; Zhu, Z. Involvement of NR1, NR2A different expression in brain regions in anxiety-like behavior of prenatally stressed offspring. Behav. Brain Res. 2013, 257, 1–7. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Pollack, M.H.; Otto, M.W. Augmentation treatment of psychotherapy for anxiety disorders with D-cycloserine. CNS Drug Rev. 2006, 12, 208–217. [Google Scholar] [CrossRef]
- Reinecke, A.; Nickless, A.; Browning, M.; Harmer, C.J. Neurocognitive processes in d-cycloserine augmented single-session exposure therapy for anxiety: A randomized placebo-controlled trial. Behav. Res. Ther. 2020, 129, 103607. [Google Scholar] [CrossRef]
- Labrie, V.; Clapcote, S.J.; Roder, J.C. Mutant mice with reduced NMDA-NR1 glycine affinity or lack of D-amino acid oxidase function exhibit altered anxiety-like behaviors. Pharmacol. Biochem. Behav. 2009, 91, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Labrie, V.; Duffy, S.; Wang, W.; Barger, S.W.; Baker, G.B.; Roder, J.C. Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice. Learn. Mem. 2009, 16, 28–37. [Google Scholar] [CrossRef]
- Pritchett, D.; Hasan, S.; Tam, S.K.; Engle, S.J.; Brandon, N.J.; Sharp, T.; Foster, R.G.; Harrison, P.J.; Bannerman, D.M.; Peirson, S.N. d-amino acid oxidase knockout (Dao(-/-)) mice show enhanced short-term memory performance and heightened anxiety, but no sleep or circadian rhythm disruption. Eur. J. Neurosci. 2015, 41, 1167–1179. [Google Scholar] [CrossRef]
- Glue, P.; Neehoff, S.; Sabadel, A.; Broughton, L.; Le Nedelec, M.; Shadli, S.; McNaughton, N.; Medlicott, N.J. Effects of ketamine in patients with treatment-refractory generalized anxiety and social anxiety disorders: Exploratory double-blind psychoactive-controlled replication study. J. Psychopharmacol. 2020, 34, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.M.; Kious, B.M. Sustained Resolution of Panic Disorder, Agoraphobia, and Generalized Anxiety Disorder With a Single Ketamine Infusion: A Case Report. Prim. Care Companion CNS Disord. 2016, 18, 27235. [Google Scholar] [CrossRef]
- Glue, P.; Medlicott, N.J.; Harland, S.; Neehoff, S.; Anderson-Fahey, B.; Le Nedelec, M.; Gray, A.; McNaughton, N. Ketamine’s dose-related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. J. Psychopharmacol. 2017, 31, 1302–1305. [Google Scholar] [CrossRef]
- Tully, J.L.; Dahlén, A.D.; Haggarty, C.J.; Schiöth, H.B.; Brooks, S. Ketamine treatment for refractory anxiety: A systematic review. Br. J. Clin. Pharmacol. 2022, 88, 4412–4426. [Google Scholar] [CrossRef]
- Zarrindast, M.R.; Nasehi, M.; Piri, M.; Heidari, N. Effects of cholinergic system of dorsal hippocampus of rats on MK-801 induced anxiolytic-like behavior. Neurosci. Lett. 2011, 505, 65–70. [Google Scholar] [CrossRef]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Boroujeni, S.N.; Lorigooini, Z.; Boldaji, F.R.; Amini-Khoei, H. Diosgenin via NMDA Receptor Exerted Anxiolytic-like Effect on Maternally Separated Mice. Curr. Pharm. Des. 2021, 27, 440–445. [Google Scholar] [CrossRef]
- Ebrahimi, C.; Gechter, J.; Lueken, U.; Schlagenhauf, F.; Wittchen, H.U.; Hamm, A.O.; Ströhle, A. Augmenting extinction learning with D-cycloserine reduces return of fear: A randomized, placebo-controlled fMRI study. Neuropsychopharmacology 2020, 45, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.H.; Landeros-Weisenberger, A.; Coughlin, C.; Mulqueen, J.; Johnson, J.A.; Gabriel, D.; Reed, M.O.; Jakubovski, E.; Bloch, M.H. Ketamine for Social Anxiety Disorder: A Randomized, Placebo-Controlled Crossover Trial. Neuropsychopharmacology 2018, 43, 325–333. [Google Scholar] [CrossRef]
- Aboujaoude, E.; Barry, J.J.; Gamel, N. Memantine augmentation in treatment-resistant obsessive-compulsive disorder: An open-label trial. J. Clin. Psychopharmacol. 2009, 29, 51–55. [Google Scholar] [CrossRef]
- Stryjer, R.; Budnik, D.; Ebert, T.; Green, T.; Polak, L.; Weizman, S.; Spivak, B. Amantadine augmentation therapy for obsessive compulsive patients resistant to SSRIs-an open-label study. Clin. Neuropharmacol. 2014, 37, 79–81. [Google Scholar] [CrossRef]
- Xu, X.; Mishra, G.D.; Jones, M. Depressive symptoms and the development and progression of physical multimorbidity in a national cohort of Australian women. Health Psychol. 2019, 38, 812–821. [Google Scholar] [CrossRef]
- Read, J.R.; Sharpe, L.; Modini, M.; Dear, B.F. Multimorbidity and depression: A systematic review and meta-analysis. J. Affect. Disord. 2017, 221, 36–46. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Wei, J.; Dilley, G.; Pittman, S.D.; Meltzer, H.Y.; Overholser, J.C.; Roth, B.L.; Stockmeier, C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 1999, 45, 1085–1098. [Google Scholar] [CrossRef]
- Kang, H.J.; Voleti, B.; Hajszan, T.; Rajkowska, G.; Stockmeier, C.A.; Licznerski, P.; Lepack, A.; Majik, M.S.; Jeong, L.S.; Banasr, M.; et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 2012, 18, 1413–1417. [Google Scholar] [CrossRef]
- Duric, V.; Banasr, M.; Stockmeier, C.A.; Simen, A.A.; Newton, S.S.; Overholser, J.C.; Jurjus, G.J.; Dieter, L.; Duman, R.S. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int. J. Neuropsychopharmacol. 2013, 16, 69–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.J.; Zheng, D.; Li, K.X.; Yang, J.M.; Pan, H.Q.; Yu, X.D.; Fu, J.Y.; Zhu, Y.; Sun, Q.X.; Tang, M.Y.; et al. Cannabinoid CB(1) receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med. 2019, 25, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Maheu, M.E.; Davoli, M.A.; Turecki, G.; Mechawar, N. Amygdalar expression of proteins associated with neuroplasticity in major depression and suicide. J. Psychiatr. Res. 2013, 47, 384–390. [Google Scholar] [CrossRef]
- Kaut, O.; Schmitt, I.; Hofmann, A.; Hoffmann, P.; Schlaepfer, T.E.; Wüllner, U.; Hurlemann, R. Aberrant NMDA receptor DNA methylation detected by epigenome-wide analysis of hippocampus and prefrontal cortex in major depression. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Z.; Wu, Z.; Chen, J.; Wang, Z.; Peng, D.; Hong, W.; Yuan, C.; Wang, Z.; Yu, S.; et al. A study of N-methyl-D-aspartate receptor gene (GRIN2B) variants as predictors of treatment-resistant major depression. Psychopharmacology 2014, 231, 685–693. [Google Scholar] [CrossRef]
- Feyissa, A.M.; Chandran, A.; Stockmeier, C.A.; Karolewicz, B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 70–75. [Google Scholar] [CrossRef]
- Karolewicz, B.; Szebeni, K.; Gilmore, T.; Maciag, D.; Stockmeier, C.A.; Ordway, G.A. Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int. J. Neuropsychopharmacol. 2009, 12, 143–153. [Google Scholar] [CrossRef]
- Karolewicz, B.; Stockmeier, C.A.; Ordway, G.A. Elevated levels of the NR2C subunit of the NMDA receptor in the locus coeruleus in depression. Neuropsychopharmacology 2005, 30, 1557–1567. [Google Scholar] [CrossRef]
- Chandley, M.J.; Szebeni, A.; Szebeni, K.; Crawford, J.D.; Stockmeier, C.A.; Turecki, G.; Kostrzewa, R.M.; Ordway, G.A. Elevated gene expression of glutamate receptors in noradrenergic neurons from the locus coeruleus in major depression. Int. J. Neuropsychopharmacol. 2014, 17, 1569–1578. [Google Scholar] [CrossRef]
- Gray, A.L.; Hyde, T.M.; Deep-Soboslay, A.; Kleinman, J.E.; Sodhi, M.S. Sex differences in glutamate receptor gene expression in major depression and suicide. Mol. Psychiatry 2015, 20, 1057–1068. [Google Scholar] [CrossRef]
- Bieler, M.; Hussain, S.; Daaland, E.S.B.; Mirrione, M.M.; Henn, F.A.; Davanger, S. Changes in concentrations of NMDA receptor subunit GluN2B, Arc and syntaxin-1 in dorsal hippocampus Schaffer collateral synapses in a rat learned helplessness model of depression. J. Comp. Neurol. 2021, 529, 3194–3205. [Google Scholar] [CrossRef] [PubMed]
- Masrour, F.F.; Peeri, M.; Azarbayjani, M.A.; Hosseini, M.J. Voluntary Exercise During Adolescence Mitigated Negative the Effects of Maternal Separation Stress on the Depressive-Like Behaviors of Adult Male Rats: Role of NMDA Receptors. Neurochem. Res. 2018, 43, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.N.; Zhang, T.; Chu, J.; Qu, N.; Lin, L.; Fang, Y.Y.; Shi, Y.; Zeng, P.; Cai, E.L.; Wang, X.M.; et al. Gender-Related Hippocampal Proteomics Study from Young Rats After Chronic Unpredicted Mild Stress Exposure. Mol. Neurobiol. 2018, 55, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Aguayo, F.I.; Aliaga, E.; Muñoz, M.; García-Rojo, G.; Olave, F.A.; Parra-Fiedler, N.A.; García-Pérez, A.; Tejos-Bravo, M.; Rojas, P.S.; et al. Chronic Stress Triggers Expression of Immediate Early Genes and Differentially Affects the Expression of AMPA and NMDA Subunits in Dorsal and Ventral Hippocampus of Rats. Front. Mol. Neurosci. 2017, 10, 244. [Google Scholar] [CrossRef]
- Zhen, L.; Shao, T.; Luria, V.; Li, G.; Li, Z.; Xu, Y.; Zhao, X. EphB2 Deficiency Induces Depression-Like Behaviors and Memory Impairment: Involvement of NMDA 2B Receptor Dependent Signaling. Front. Pharmacol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Francija, E.; Petrovic, Z.; Brkic, Z.; Mitic, M.; Radulovic, J.; Adzic, M. Disruption of the NMDA receptor GluN2A subunit abolishes inflammation-induced depression. Behav. Brain Res. 2019, 359, 550–559. [Google Scholar] [CrossRef]
- Trullas, R.; Skolnick, P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur. J. Pharmacol. 1990, 185, 1–10. [Google Scholar] [CrossRef]
- Zarate, C.A., Jr.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, Y.L.; Liu, W.J.; Wang, C.Y.; Zhan, Y.N.; Li, H.Q.; Chen, L.J.; Li, M.D.; Ning, Y.P. Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. J. Psychiatr. Res. 2018, 106, 61–68. [Google Scholar] [CrossRef]
- Singh, J.B.; Fedgchin, M.; Daly, E.J.; De Boer, P.; Cooper, K.; Lim, P.; Pinter, C.; Murrough, J.W.; Sanacora, G.; Shelton, R.C.; et al. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. Am. J. Psychiatry 2016, 173, 816–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanos, P.; Gould, T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Swainson, J.; Thomas, R.K.; Archer, S.; Chrenek, C.; MacKay, M.A.; Baker, G.; Dursun, S.; Klassen, L.J.; Chokka, P.; Demas, M.L. Esketamine for treatment resistant depression. Expert Rev. Neurother. 2019, 19, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Jelen, L.A.; Young, A.H.; Stone, J.M. Ketamine: A tale of two enantiomers. J. Psychopharmacol. 2021, 35, 109–123. [Google Scholar] [CrossRef]
- Lauterbach, E.C. Dextromethorphan as a potential rapid-acting antidepressant. Med. Hypotheses 2011, 76, 717–719. [Google Scholar] [CrossRef]
- De Martin, S.; Gabbia, D.; Folli, F.; Bifari, F.; Fiorina, P.; Ferri, N.; Stahl, S.; Inturrisi, C.E.; Pappagallo, M.; Traversa, S.; et al. REL-1017 (Esmethadone) Increases Circulating BDNF Levels in Healthy Subjects of a Phase 1 Clinical Study. Front. Pharmacol. 2021, 12, 671859. [Google Scholar] [CrossRef]
- Fava, M.; Stahl, S.; Pani, L.; De Martin, S.; Pappagallo, M.; Guidetti, C.; Alimonti, A.; Bettini, E.; Mangano, R.M.; Wessel, T.; et al. REL-1017 (Esmethadone) as Adjunctive Treatment in Patients With Major Depressive Disorder: A Phase 2a Randomized Double-Blind Trial. Am. J. Psychiatry 2022, 179, 122–131. [Google Scholar] [CrossRef]
- Zarate, C.A., Jr.; Mathews, D.; Ibrahim, L.; Chaves, J.F.; Marquardt, C.; Ukoh, I.; Jolkovsky, L.; Brutsche, N.E.; Smith, M.A.; Luckenbaugh, D.A. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol. Psychiatry 2013, 74, 257–264. [Google Scholar] [CrossRef]
- Nagele, P.; Duma, A.; Kopec, M.; Gebara, M.A.; Parsoei, A.; Walker, M.; Janski, A.; Panagopoulos, V.N.; Cristancho, P.; Miller, J.P.; et al. Nitrous Oxide for Treatment-Resistant Major Depression: A Proof-of-Concept Trial. Biol. Psychiatry 2015, 78, 10–18. [Google Scholar] [CrossRef]
- Heresco-Levy, U.; Javitt, D.C.; Gelfin, Y.; Gorelik, E.; Bar, M.; Blanaru, M.; Kremer, I. Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J. Affect. Disord. 2006, 93, 239–243. [Google Scholar] [CrossRef]
- Burgdorf, J.; Zhang, X.L.; Nicholson, K.L.; Balster, R.L.; Leander, J.D.; Stanton, P.K.; Gross, A.L.; Kroes, R.A.; Moskal, J.R. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 2013, 38, 729–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moskal, J.R.; Burch, R.; Burgdorf, J.S.; Kroes, R.A.; Stanton, P.K.; Disterhoft, J.F.; Leander, J.D. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin. Investig. Drugs 2014, 23, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.; Macaluso, M.; Mehra, D.O.; Zammit, G.; Moskal, J.R.; Burch, R.M. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 2015, 21, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.S.; Li, C.Y.; Yang, X.C.; Fang, J.; Yang, Y.X.; Guo, J.Y. Protective effect of gan mai da zao decoction in unpredictable chronic mild stress-induced behavioral and biochemical alterations. Pharm. Biol. 2010, 48, 1328–1336. [Google Scholar] [CrossRef]
- Shin, I.J.; Son, S.U.; Park, H.; Kim, Y.; Park, S.H.; Swanberg, K.; Shin, J.Y.; Ha, S.K.; Cho, Y.; Bang, S.Y.; et al. Preclinical evidence of rapid-onset antidepressant-like effect in Radix Polygalae extract. PLoS ONE 2014, 9, e88617. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, F.; Fu, Z.W.; Zhang, B.; Huang, C.G.; Li, Y. Timosaponin derivative YY-23 acts as a non-competitive NMDA receptor antagonist and exerts a rapid antidepressant-like effect in mice. Acta Pharmacol. Sin. 2016, 37, 166–176. [Google Scholar] [CrossRef]
- Ren, L.; Chen, G. Rapid antidepressant effects of Yueju: A new look at the function and mechanism of an old herbal medicine. J. Ethnopharmacol. 2017, 203, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Kao, C.F.; Tsai, S.J.; Li, C.T.; Lin, W.C.; Hong, C.J.; Bai, Y.M.; Tu, P.C.; Su, T.P. Treatment response to low-dose ketamine infusion for treatment-resistant depression: A gene-based genome-wide association study. Genomics 2021, 113, 507–514. [Google Scholar] [CrossRef]
- Tiger, M.; Veldman, E.R.; Ekman, C.J.; Halldin, C.; Svenningsson, P.; Lundberg, J. A randomized placebo-controlled PET study of ketamine’s effect on serotonin(1B) receptor binding in patients with SSRI-resistant depression. Transl. Psychiatry 2020, 10, 159. [Google Scholar] [CrossRef]
- Murrough, J.W.; Wade, E.; Sayed, S.; Ahle, G.; Kiraly, D.D.; Welch, A.; Collins, K.A.; Soleimani, L.; Iosifescu, D.V.; Charney, D.S. Dextromethorphan/quinidine pharmacotherapy in patients with treatment resistant depression: A proof of concept clinical trial. J. Affect. Disord. 2017, 218, 277–283. [Google Scholar] [CrossRef]
- Guimarães, M.C.; Guimarães, T.M.; Hallak, J.E.; Abrão, J.; Machado-de-Sousa, J.P. Nitrous oxide as an adjunctive therapy in major depressive disorder: A randomized controlled double-blind pilot trial. Braz. J. Psychiatry 2021, 43, 484–493. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Halberstam, B.; Gangwisch, J. Single-dose ketamine followed by daily D-Cycloserine in treatment-resistant bipolar depression. J. Clin. Psychiatry 2015, 76, 737–738. [Google Scholar] [CrossRef]
- Park, L.T.; Kadriu, B.; Gould, T.D.; Zanos, P.; Greenstein, D.; Evans, J.W.; Yuan, P.; Farmer, C.A.; Oppenheimer, M.; George, J.M.; et al. A Randomized Trial of the N-Methyl-d-Aspartate Receptor Glycine Site Antagonist Prodrug 4-Chlorokynurenine in Treatment-Resistant Depression. Int. J. Neuropsychopharmacol. 2020, 23, 417–425. [Google Scholar] [CrossRef]
- Preskorn, S.H.; Baker, B.; Kolluri, S.; Menniti, F.S.; Krams, M.; Landen, J.W. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharmacol. 2008, 28, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Coyle, J.T. Glutamate hypothesis in schizophrenia. Psychiatry Clin. Neurosci. 2019, 73, 204–215. [Google Scholar] [CrossRef]
- Jorratt, P.; Hoschl, C.; Ovsepian, S.V. Endogenous antagonists of N-methyl-d-aspartate receptor in schizophrenia. Alzheimers Dement. 2021, 17, 888–905. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Bian, L.; Yin, Y.; Guo, J. Targeting NMDA Receptors in Emotional Disorders: Their Role in Neuroprotection. Brain Sci. 2022, 12, 1329. https://doi.org/10.3390/brainsci12101329
Wang S, Bian L, Yin Y, Guo J. Targeting NMDA Receptors in Emotional Disorders: Their Role in Neuroprotection. Brain Sciences. 2022; 12(10):1329. https://doi.org/10.3390/brainsci12101329
Chicago/Turabian StyleWang, Siqi, Lihua Bian, Yi Yin, and Jianyou Guo. 2022. "Targeting NMDA Receptors in Emotional Disorders: Their Role in Neuroprotection" Brain Sciences 12, no. 10: 1329. https://doi.org/10.3390/brainsci12101329